To give the Binkhorst Medal Lecture is without doubt one of the greatest honors that can be bestowed on an ophthalmologist. Cornelius D. Binkhorst, MD, for whom the lecture was named, was a giant in our specialty. Having tried the IOL designed by Sir Harold Ridley soon after its development, Binkhorst realized that its main shortcomings related to its weight and its ability to remain stable in the eye. He therefore designed his four-loop iris-fixated IOL for use with intracapsular surgery; this was the first IOL to produce consistent results.
Binkhorst also recognized that, if he could use the lens capsule to support the IOL, this would give even better centration and stability, and thus he advocated a return to extracapsular cataract surgery (ECCE). I was privileged to assist Dr. Binkhorst at surgery during a conference held at Charing Cross Hospital in London in 1980.
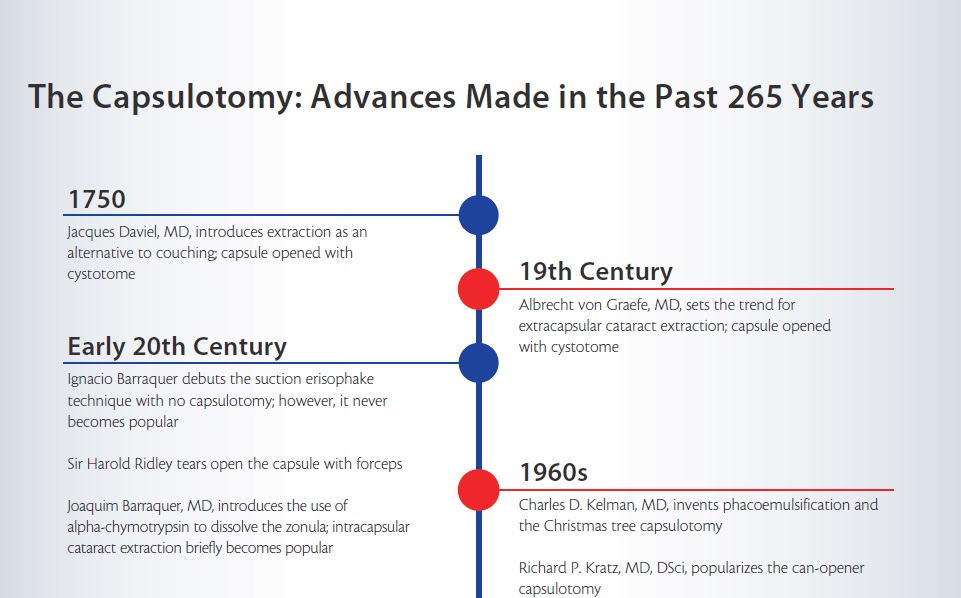
The subject of my Binkhorst lecture relates to the capsule—specifically to the methods of opening it to remove the cataractous lens nucleus and how these have changed over the centuries (http://eyetube.net/?v=erejo). The capsular opening has evolved, first from a roughly made tear to allow access to the nucleus for extraction, to the creation of more regular openings to allow support for IOLs, then to a continuous circular tear to help contain the IOL, and most recently to precision sizing and location with laser and other technologies. Although this opening is usually called a capsulotomy, the term capsulectomy should more correctly be used because the tissue is normally removed in modern surgery.
At a Glance
• Over the course of 265 years, the capsulotomy has evolved from a crude opening to give access to the nucleus for its removal to a continuous curvilinear capsulorrhexis for both anterior and posterior capsules to contain the IOL.
• The capsular opening is usually called a capsulotomy; however, the term capsulectomy should more correctly be used because the tissue is normally removed in modern surgery.
• Although many surgeons may pride themselves on their ability to perform a manual capsulorrhexis, this may soon become a historical skill.
THE STORY BEGINS
The story begins with the father of modern cataract surgery, the French surgeon Jacques Daviel, MD. Not happy with the couching technique (lens depression) that had been used for millennia, in 1750 he devised a method of cataract surgery that involved an inferior incision in the eye (Figure 1). The capsule was opened with a cystotome, and the surgeon expressed the nucleus from the eye by depressing the globe with his fingers.

Figure 1. Daviel’s capsulotomy technique involved an inferior incision in the eye.
The introduction of extraction as an alternative to couching caused much controversy, particularly in Britain, where prominent surgeon Percival Pott condemned it as “a kind of fashion.” Others such as Samuel Sharp were more enthusiastic, and extraction eventually became the method of choice in this and other parts of the world. The great 19th-century surgeon Albrecht von Graefe, MD, designer of a special long-bladed cataract incision knife, set the trend for ECCE for the next century. He, too, used a cystotome very much like that of Daviel.
At the beginning of the 20th century, Ignacio Barraquer devised a means of removing the cataract without the need for a capsulotomy. However, his suction erisophake technique (Figure 2) did not gain popularity. The next significant development belongs to Ridley, the inventor of the IOL. In his ECCE technique, begun with a Graefe knife and incision, he used a pair of forceps to tear open the capsule (Figure 3). His major advance was to design the first IOLs. Unlike von Graefe and Barraquer, he could close the wound with a stitch.
For a brief period in the 20th century, following the introduction of alpha-chymotrypsin to dissolve the zonula by Joaquin Barraquer, MD, intracapsular cataract surgery became the usual method for nucleus removal. Binkhorst tried to reintroduce ECCE for the reasons already mentioned, and he devised a series of shapes for the capsular opening to hold his two-loop IOL.

Figure 2. Ignacio Barraquer removing a cataract with the erisophake technique.
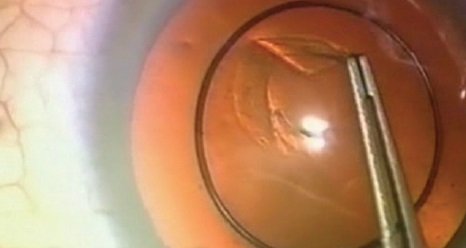
Figure 3. Sir Harold Ridley performing a forceps capsulotomy.
PHACO AND AFTER
At the end of the 1960s, a new type of ECCE was being developed that was destined to change practices for the foreseeable future. This was the invention of phacoemulsification by Charles D. Kelman, MD. In order to open the capsule for ultrasonic disassembly of the nucleus, Kelman devised the hooked cystotome, using it to perform what he called a Christmas tree capsulotomy by dragging the cystotome across the front of the lens to create a large opening (Figure 4A).
Initially Kelman performed emulsification of the nucleus in the posterior chamber, but he found that it was more safely performed in the anterior chamber. This maneuver required the surgeon to prolapse the nucleus from the capsular bag into the anterior chamber. Without a large capsulotomy this was difficult, and, thus, Kelman enhanced the Christmas tree with added capsular tears.
Richard P. Kratz, MD, DSci, one of the early adopters and modifiers of Kelman’s technique, believed that phacoemulsification of the nucleus should be done away from the cornea. He devised an iris plane technique and, in order to make the capsular opening as symmetrical as possible, changed the way the opening was made. He advocated using multiple small cuts in the capsule to create a can-opener capsulotomy (Figure 4B). When I first started performing phacoemulsification in 1979, this is what I learned.
Watch it Now
Dr. Packard discusses the evolution of the capsulotomy over 265 years, from the first extraction of a cataractous lens to recent advances in femtosecond laser technology and beyond.
Meanwhile, new devices to create a capsulotomy were being tried. Daniele S. Aron Rosa, MD, PhD, the inventor of Nd:YAG laser posterior capsulotomy, also tried to use this instrument for opening the anterior capsule prior to cataract surgery (Figure 4C). It certainly cut the capsule; however, unless surgery was carried out quickly, a huge rise in IOP resulted. This practice was quickly abandoned.
In the early days, openings in the capsule had been made simply to gain access to the lens nucleus for its removal. IOLs changed that. In 1983, with the arrival of new posterior chamber IOLs from surgeons such as Steven P. Shearing, MD; Robert M. Sinskey, MD; and Dr. Kratz, there was a debate at the American Academy of Ophthalmology (AAO) meeting to decide whether it was better to place these lenses in the capsular bag or in the ciliary sulcus. The problem was that the can-opener capsulotomy could not guarantee the IOL would stay where it was put or even whether it was in the capsular bag or the sulcus.
Several solutions were put forth. Albert Galand, MD, PhD, an advocate of ECCE, designed his envelope technique in order to make more certain that the IOL was in the capsular bag (Figure 4D). He created a linear slit in the proximal capsule to allow the nucleus to be expressed. After the IOL was inserted, a scissor cut was made, and the capsule was torn open with forceps.
EVOLUTION OF THE CAPSULOTOMY IN PICTURES
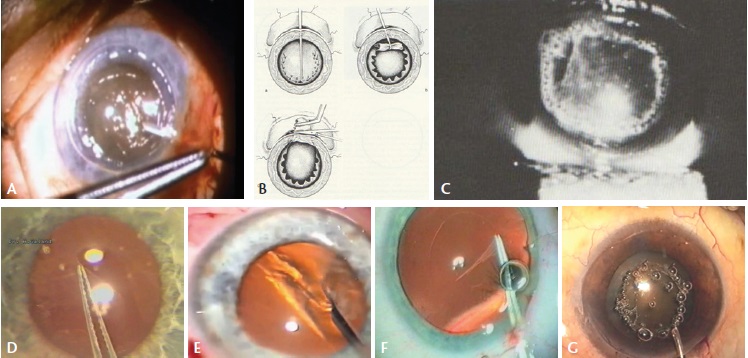
Figure 4. Charles D. Kelman, MD, performing a Christmas tree capsulotomy (A); can-opener capsulotomy (B); Nd:YAG laser anterior capsulotomy (C); Albert Galand completing a capsulotomy with the envelope technique (D); Calvin K. Fercho, MD, performing a circular capsulotomy (E); the author performing a posterior CCC (F); Kloti high-frequency radio diathermy capsulotomy (G).
CAPSULORRHEXIS BRINGS MAJOR CHANGES, SOME CHALLENGES
All uncertainties about the stability of the IOL would change with the introduction of the continuous curvilinear capsulorrhexis (CCC). Two ophthalmologists, Thomas Neuhann, MD, and Howard V. Gimbel, MD, MPH, FRCSC, FACS, are generally given credit for co-inventing this technique. However, Kimiya Shimizu, MD, PhD, in Japan was also devising his own method of doing this at the same time in the mid-1980s. All three surgeons had realized that a continuous circular tear would be fundamentally stronger than anything that had been tried before and that this capsulotomy would guarantee the IOL could be placed in the capsular bag and remain there.
During the course of preparing for the Binkhorst Lecture, I. Howard Fine, MD, told me that, in fact, another surgeon preceded the three cited above with development of a circular tear capsulotomy. Calvin K. Fercho, MD, who spent his career in Fargo, North Dakota, started using his technique in the late 1970s (Figure 4E). Owing to a long illness, however, he did not present the technique until 1986. A number of surgeons confirmed that they saw him doing this circular tear technique in the early 1980s, and he should be given credit for being the first.
With the introduction of the capsulorrhexis, the IOL could be placed reliably and securely in the capsular bag. On the downside, however, previously developed phaco techniques, such as the Kratz technique requiring the nucleus to be tilted, became more difficult. As a result, multiple phaco techniques were developed to overcome these difficulties: chip-and-flip, divide-and-conquer, nucleofractis, phaco chop, and phaco prechop. These were all methods of physically breaking the nucleus within the capsular bag to assist its removal.

Figure 5. The author using trypan blue dye for the first time.
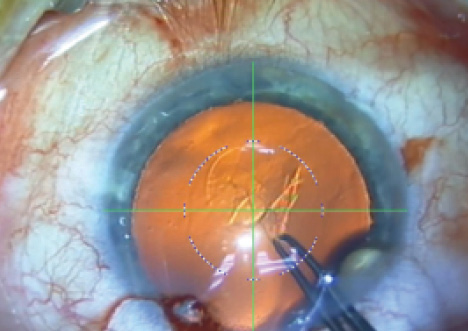
Figure 6. Heads-up display on the Verion for capsulotomy.
One challenge with any form of ECCE is posterior capsular opacification (PCO), particularly for long-term IOL implantation in children. Dr. Gimbel suggested that creating a posterior capsulorrhexis and capturing the IOL optic in it might overcome this problem (Figure 4F). The idea of using the capsule to capture the IOL would later be used in other ways.
Another problem that would become apparent with the CCC was that, although a good red reflex made it easier, a white cataract made it challenging. One attempt to deal with this was the development of the Kloti high-frequency radio diathermy device (Figure 4G). Around 1999, this device became largely unnecessary with the use of the vital dye trypan blue to stain the capsule. Although my initial research suggested that Dutch surgeon Gerrit R.J. Melles, MD, PhD, was the first to stain the capsule with trypan blue dye in 1999, it appears that Minas Coroneo, MD, from Australia, had an earlier patent for the same method. Coroneo developed the technique when operating on patients with very mature cataracts in a remote area of Australia. With use of the dye, every capsule could now be visualized for capsulorrhexis (Figure 5).

GREATER PRECISION
Once surgeons could make the capsulotomy in most eyes reliably, attention turned to making the results more precise. Marie-José Tassignon, MD, PhD, FEBO, developed a ring-shaped caliper, intended to be centered on the capsule, to help make the size and centration of capsulorrhexis more accurate (http://eyetube.net/?v=sonidi). Another approach is to indent the cornea with a circular guide. More recently, high-tech devices such as the Verion (Alcon; Figure 6) and Callisto eye (Carl Zeiss Meditec) provide heads-up displays in the microscope oculars to guide capsulorrhexis creation. These devices project a circular image onto the capsule to assist the surgeon.
But why are we concerned about the size and centration of the capsulotomy? For starters, IOL centration and stability are particularly important for the proper function of multifocal and toric IOLs. Additionally, one of the issues in obtaining accurate visual outcomes from biometry is predicting the IOL’s postoperative effective lens position (ELP). It is thought that more accurate capsulorrhexis sizing and positioning should facilitate this. Further, proper overlapping of the IOL edge with the capsule has been shown to be important in creating a shrink-wrapping of the IOL by the capsule to lessen PCO.
If we can make the capsulorrhexis more accurately, can we then use the capsule edge to hold the IOL? Professor Tassignon designed a lens specifically to be held in place by the anterior and posterior capsules. The bag-in-the-lens technique—and the Bag-in-the-Lens IOL (Morcher) developed for use with the technique—precludes any possibility of PCO. However, getting the centration and sizing of the capsular openings right requires considerable manual skills. More recently, Samuel Masket, MD, designed a lens (ND IOL; Morcher) to be inserted inside an anterior capsulotomy created with a femtosecond laser. This lens is meant to minimize the negative dysphotopsias sometimes seen with sharp-edged hydrophobic IOLs. Similar to the Bag-in-the-Lens, the edge of the anterior capsule is trapped in a groove at the edge of the ND IOL.
Watch it Now
Dr. Tassignon illustrates techniques employing a ring caliper to guide capsulorrhexis. The device is introduced to achieve the desired anterior rhexis size, shape, and centration.
LASER CAPSULOTOMY
Having now mentioned the femtosecond laser, let us consider its place in the story of the capsulotomy. In 2008, Zoltan Z. Nagy, MD, carried out the first cataract procedure assisted by the use of a femtosecond laser. The addition of the laser meant that certain things could now become reality. For the first time, truly circular capsulotomies of a given size and in a given position could be made with no risk of tearing during creation and without the variables of manual techniques.
There are caveats attached to the use of the femtosecond laser in cataract surgery, however. First, because of the size of the laser, a second room may be needed for its use. This can interfere with the surgical flow of high-volume surgeons. Second, the acquisition costs of these devices are high, as are the ongoing costs of annual service and patient interface kits. Third, the patient must pay a significant premium to make use of this technology. As yet, the advantages are not clear in terms of achieving better outcomes with the laser; however, as the technology is continually developing, this may change.
There is no doubt that the femtosecond laser makes more circular and accurately sized capsulotomies compared with any manual technique. However, the smoothness of the capsulotomy edge has been a subject of concern. Brendan J. Vote, MBBS, FRANZCO, and colleagues have used scanning electron microscopy to look at laser capsulotomy and manual capsulorrhexis edges, and the latter have much smoother edges (Figure 7).1 This is mainly because the femtosecond laser is a pulsed laser, and the multiple redundant shots make for a serrated edge. In a study by Abell et al, the incidence of tears during surgery with a complete capsulotomy was significantly higher with laser than with manual capsulorrhexis.2 This was thought to be due to the way the edge was created by the laser.
Still, the majority of those who use femtosecond lasers for cataract surgery value capsulotomy creation above any other function of the device. Might there be another way of achieving the same consistency and accuracy? Recently, a thermal laser approach has been developed. When the anterior capsule is stained with trypan blue, the CapsuLaser (CapsuLaser), which operates in continuous mode, can be moved in a circular manner to open the capsule in a chosen position and at a chosen size. The collagen of the capsule is converted to amorphous collagen, which makes the edge elastic and strong. In terms of circularity, the thermal laser openings compare well with femtosecond laser openings (data on file with CapsuLaser). The device is small and fits onto the operating microscope; it does not interfere with normal surgical flow.
Preliminary clinical results using this device with 2-month follow-up have been reported.3 No pupil constriction was seen after laser use, there was no untoward anterior chamber activity postoperatively, and all corneas were clear with endothelial counts as expected. The capsulotomies were well centered, and, at 2 months, they had not contracted and there was no change in IOL position. The edges of capsulotomies made with the CapsuLaser were much smoother than those seen with some femtosecond lasers.

Figure 7. Scanning electron microscopy (SEM) of femtosecond capsulotomy edge (A); SEM of manual capsulotomy edge (B).
OTHER APPROACHES
The CapsuLaser device is discussed elsewhere in this issue, along with two other devices, the Zepto capsulotomy system (Mynosys) and ApertureRx Precision Capsulotomy System (International BioMedical Devices). These two devices use metallic thermal circular tips applied to the capsule through the phaco incision to cut a circular capsular opening. No clinical results have been reported in the literature yet, but extensive animal and cadaver eye work has been carried out (personal communication with employees of Mynosys and International BioMedical Devices).
Ophthalmologists should thus soon have a range of options available to make more circular, predictably sized, and well-positioned capsulotomies. Lens manufacturers are already beginning to develop IOLs to take advantage of this trend, and hopefully these will allow more accurate ELP and, thus, achievement of better visual outcomes and predictability.
SUMMARY
The capsulotomy has come a long way in the past 265 years, from a crude opening to give access to the nucleus for its removal; through various iterations of cystotomes and forceps to create different shapes of opening for IOL support; to the CCC for both anterior and posterior capsules to contain the IOL.
Soon, as presented in this issue of CRST Europe, we will hopefully have many devices—from lasers to metallic thermal instruments—to create perfectly round, consistent, well-centered capsulotomies. IOLs are being developed to take advantage of this and provide better centration and ELP prediction. Although many surgeons may pride themselves on their ability to perform a manual capsulorrhexis, this may soon become a historical skill.
1. Vote B. Capsulotomy integrity after femtosecond laser cataract surgery. Video presented at: the XXXII Congress of the ESCRS; September 13-17, 2014; London.
2. Abell RG, Darian-Smith E, Kan JB, et al. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: Outcomes and safety in more than 4000 cases at a single center. J Cataract Refract Surg. 2015;41(1):47-52.
3. Stodulka P. Laser capsulotomy: Simple, fast, cost effective—First experience with a new laser. Paper presented at: the XXXIII Congress of the ESCRS; September 5-9, 2015; Barcelona, Spain.
Richard Packard, MD, FRCS, FRCOphth
• Prince Charles Eye Unit, King Edward VII Hospital, Windsor, England
• mail@eyequack.vossnet.co.uk
• Financial disclosure: Consultant, Equity shareholder (CapsuLaser)