The Case for a Manual Capsulorrhexis

A smooth, strong edge; no expensive equipment; helpful tactile feedback: What’s not to like?
By Richard R. Schulze Jr, MPhil (Oxon), MD
In the early 1980s, Gimbel and Neuhann simultaneously and independently developed the continuous curvilinear capsulorrhexis (CCC),1 a quantum leap over previous methods such as the can-opener technique used by cataract surgeons to open the anterior capsule for extracapsular cataract surgery. The development of the CCC reduced the frequency of anterior capsular tears extending to the bag equator with a consequent reduction in dropped nuclei and other common complications of the time. Consistent capsulorrhexis also allowed development of IOLs specifically to be placed within the capsular bag and not the ciliary sulcus, providing greater stability over time and more predictable refractive outcomes.
Since Nagy’s first report of the use of femtosecond lasers in cataract surgery in 2009,2 much attention has been focused upon the ability of the femtosecond laser to create a round capsular opening. There is little doubt that the computer-guided laser can create a more consistently round capsulotomy than can a surgeon using manual methods.3 Initial speculation suggested that the increased precision with which the laser can create a rounder capsulotomy (as compared with manual CCC) would lead to greater accuracy in refractive outcomes due to an improvement in predictability of the effective lens position (ELP).
But do these claims for improved refractive outcomes because of a more precise capsular opening hold water?
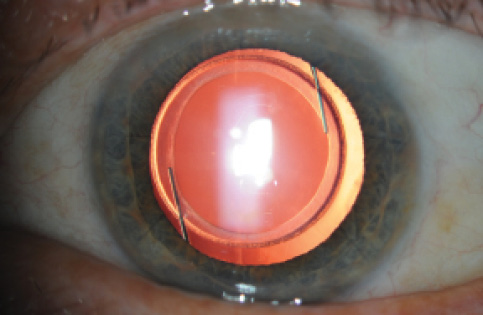
Figure 1. A well-centered manual capsulorrhexis.
The ophthalmic industry has spent several billions of dollars developing femtosecond laser technology and promoting it to surgeons and the public, but results thus far suggest no clear benefit to the patient in terms of refractive outcomes. In fact, the best prospective study looking at refractive outcomes after laser-assisted cataract surgery (LACS) and routine phacoemulsification found that the phaco group had tighter outcomes with fewer refractive outliers.4 Aesthetically, all of us like to see a round, well-centered capsular opening with complete overlap of the optic (Figure 1), whether it was produced manually or with a laser. Certainly I aim to make a perfect capsulorrhexis in every case, and this is one of the main factors I look at when I try to judge the quality of my own surgical performance.
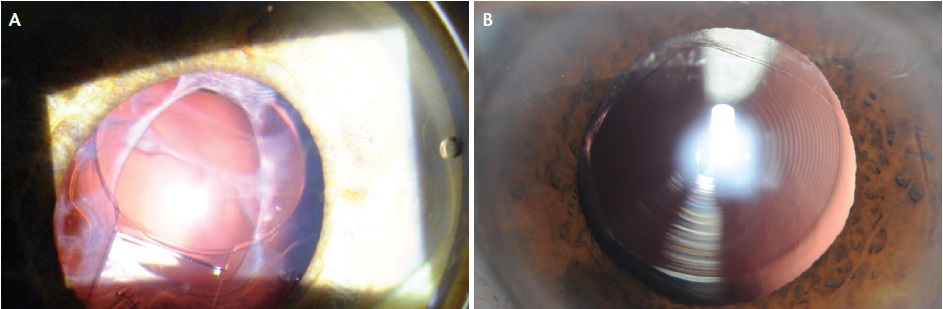
Figure 2. Capsular phimosis in a patient shortly after cataract surgery with femtosecond laser assistance and implantation of a Crystalens (Bausch + Lomb; A). Femtosecond capsulotomy 7 months postoperatively. Note the lack of circularity and irregular fibrosis at the margin (B).
THE DETERMINING FACTOR
Laser manufacturers would have us believe that a well-centered, round capsular opening with perfect optic overlap is a necessary condition for a predictable refractive outcome, and these companies are heavily invested in the idea of convincing surgeons to purchase lasers costing half a million dollars to achieve this, with income streams to the laser companies of several multiples of the purchase price. With regard to the effect of the laser capsulotomy on refractive outcomes, however, I am aware of at least three studies that come to the opposite conclusion: the capsulotomy is not the determining factor in refractive outcomes.5-7
Study No. 1: Davidorf et al.5 In this study of 175 eyes, the authors found no statistically significant difference in refractive outcomes in eyes with excellent capsule overlap compared with those with less than perfect capsulotomies.
Study No. 2: Findl et al.6 This group also found no effect in refractive outcomes with use of the femtosecond laser. According to the authors, “the supposed advantage of the more predictable capsulorrhexis achieved with [LACS] may be based on a false assumption about its influence on the stability of an IOL’s position and orientation.” Moreover, “the rhexis effect is weak, and that brings into question whether femtosecond laser will actually result in a better and more predictable IOL positioning.”
Study No. 3: Davison et al.7 In this study of more than 2,000 eyes, looking at the effect of capsule overlap (or lack thereof) onto the optic, Davison and colleagues found that “imperfection of optic overlap had no anatomic or refractive clinical significance.”
Moreover, the femtosecond laser capsulotomy does not stay round over time. Therefore, even if one accepts the argument that the circularity of the capsulotomy is important initially, whatever effect it may have had tends to diminish (Figure 2).

Figure 3. The margin of a femtosecond capsulotomy. Note the serrated edge.
Nagy’s landmark paper on LACS in 2009 included scanning electron micrographs (SEMs) of porcine capsular openings made manually and by the laser.2 He concluded that the margin of the femtosecond laser capsulotomy was “at least as smooth” as the manual capsulorrhexis. Crucially, however, the magnification of the SEMs in the study was only 300X. Subsequent studies by Abell et al,7 Bala et al,8 and Mastropasqua et al,3 all with SEMs at higher magnification, showed that the femtosecond capsulotomy margin is not as smooth as a manual rhexis. When one sees the capsule margin at appropriately higher magnification (1,000–10,000X), one can appreciate that the femtosecond capsulotomy is more like an old-fashioned can-opener capsulotomy (Figure 3).
WHY MANUAL IS THE GOLD STANDARD
• The smooth edge of the manual capsulorrhexis, as documented on repeated SEM studies, is stronger and more resistant to tears than that of the laser capsulotomy.
• A manual capsulorrhexis can be performed inexpensively and quickly, either with a bent needle cystotome or intraocular forceps, and there is no additional cost to the patient.
• Behavior of the capsule during capsulorrhexis provides tactile and visual clues that serve to gauge the strength (or lack thereof) of the zonules, thereby guiding subsequent steps in the procedure.
• Refractive outcomes are as good as or better than those with femtosecond laser capsulotomy, even if the capsule is not Figure 1. A well-centered manual capsulorrhexis. as round initially.
Abell et al also documented a higher complication rate in terms of radial anterior tears with femtosecond laser use. In their prospective, multicenter study, the incidence of radial tears was 15 in 804 cases (seven of which extended to the posterior capsule) in the femtosecond laser group compared with one radial tear in 822 manual cases. When one looks at the smooth edge of a manual capsulorrhexis under SEM (Figure 4), it is easy to understand why the femtosecond capsulotomy is weaker and more prone to tearing.
The findings of Abell et al showing higher complication rates with femtosecond laser were borne out by the ESCRS/EUREQUO FLACS study, which also reported a higher complication rate with femtosecond capsulotomy (3.2%) as compared with manual capsulorrhexis (1.8%).10 It is important for the reader to note that the studies by Abell and colleagues and by the ESCRS were not industry sponsored. Further, recent reports have found increased inflammation, apoptosis, and prostaglandin release to be associated with femtosecond capsulotomies.11,12
In summary of these studies, what have we gained with the advent of the femtosecond capsulotomy?
- There is no improvement in refractive outcomes;4-7
- The capsule margin is weaker, not stronger;3,8,9
- There is a higher incidence of radial tears;8
- The femtosecond laser capsulotomy releases prostaglandins associated with increased inflammation and apoptosis, which may explain early anecdotal reports of increased capsular phimosis and lens epithelial cell overgrowth;11,12
- The laser cannot be used to create capsulotomies in eyes with small pupils, and the prostaglandin release with femtosecond laser use may cause miosis;11,12 and
- There is a huge increase in cost for the patient without measurable benefit.13
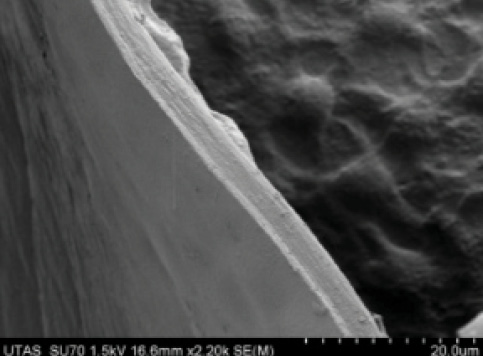
Figure 4. The smooth margin of a manual capsulorrhexis is notable on this SEM.
Moreover, one factor that has sometimes been lost in the femtosecond versus manual debate is that the tactile response of the capsule during manual capsulorrhexis is a useful indicator of the strength (or lack thereof) of the zonules, thus enabling the surgeon to proceed accordingly through later steps of the case. With no tactile engagement of the capsule during laser capsulotomy, the femtosecond surgeon is at a disadvantage compared with the manual capsulorrhexis surgeon.
CONCLUSION
For the four reasons listed in Why Manual is the Gold Standard, on the previous page, manual capsulorhexis remains the standard of care in cataract surgery. When femtosecond lasers can provide results that surpass phacoemulsification with manual capsulorrhexis, I will be more than happy to use them, but, in my opinion, the use of current femtosecond lasers to perform capsulotomy is a step backward, not forward.
1. Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16:31-37.
2. Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. J Refract Surg. 2009;25(12):1053-1060.
3. Mastropasqua L, Toto L, Calienno R, et al. Scanning electron microscopy evaluation of capsulorhexis in femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(10):1581-1586.
4. Lawless M, Bali SJ, Hodge C, Roberts TV, Chan C, Sutton G. Outcomes of femtosecond laser cataract surgery with a diffractive multifocal intraocular lens. J Refract Surg. 2012;28(12):859-864.
5. Davidorf J. The “Ideal” Capsulorhexis—Does it Matter? Eyenet. 2012;11:23-24.
6. O’hEineachain R. Study calls into question benefit of femto-cataract surgery’s accuracy in capsulorrhexis. Eurotimes. September 2013.
7. Davison JA. Intraoperative capsule complications during phacoemulsification and IOL implantation. Paper presented at: the 2012 Annual ASCRS Meeting; April 20-24, 2012; Chicago.
8. Abell RG, Davies PEJ, Phelan D, Goemann K, McPherson ZE, Vote BJ. Anterior capsulotomy integrity after femtosecond laser-assisted cataract surgery. Ophthalmology. 2014;121:17-24.
9. Bala C, Xia Y, Meades K. Electron microscopy of laser capsulotomy edge: Interplatform comparison. J Cataract Refract Surg. 2014;40(8):1382-1389.
10. Barry P. ESCRS/EUREQUO FLACS Study. Paper presented at: European Society of Cataract and Refractive Surgeons Annual Meeting; September 13-17, 2014; London.
11. Toto L, Calienno R, Curcio C, et al. Induced inflammation and apoptosis in femtosecond laser-assisted capsulotomies and manual capsulorhexes: an immunohistochemical study. J Refract Surg. 2015;31(5):290-294.
12. Schultz T, Joachim SC, Stellbogen M, Dick HB. Prostaglandin release during femtosecond laser-assisted cataract surgery: main inducer. J Refract Surg. 2015;31(2):78-81.
13. Abell RG, Vote BJ. Cost-effectiveness of femtosecond laser-assisted cataract surgery versus phacoemulsification cataract surgery. Ophthalmology. 2014;121(1):10-16.
Laser Capsulotomy Brings Advantages to Cataract Surgery

Together with a specially designed IOL, the procedure ushers in a new era of cataract surgery.
By Detlef Holland, MD
My positive impression of LACS has increased continually since I started to perform the procedure 3 years ago. The demands of our patients for better refractive outcomes and the safest possible procedure are always rising, and surgeons must respond to these wishes. For us, refractive LACS is the best way to achieve these goals.
Although traditional phaco techniques offer good results, there are still potential disadvantages due to variability in surgeons’ skills and fluctuations in daily performance. In this respect, the laser has an advantage because it is not subject to these variables.
DISADVANTAGES OF A MANUAL METHOD
Especially in capsulotomy creation, the surgeon maintains great influence on the surgical result. Decentration, tilt, shift, or induction of aberrations with unintended refractive results may be caused by an imperfect capsulorrhexis, which may be too large, too small, or decentered with asymmetric overlap of the IOL optic.1-4 Other capsulotomy-related problems can include capsular phimosis or the shrink-wrap effect due to an undersized capsulotomy.5,6 Major problems can also be caused by a tear in the capsule that runs posteriorly and results in vitreous loss, a dropped lens, and/or the need for a sulcus-fixated IOL.5,6
AT A GLANCE
• Dr. Schulze argues that, with no tactile engagement of the capsule during laser capsulotomy, the femtosecond surgeon could be at a disadvantage compared with the manual capsulorrhexis surgeon. On the other hand, Dr. Holland argues that LACS may have an advantage over phaco techniques because it is free of fluctuating factors including variability in surgeons’ skills and inconsistencies in daily performance.
• Studies suggest that refractive outcomes in manual capsulorrhexis are as good as or better than those with femtosecond laser capsulotomy.
• One interesting side effect of the introduction of laser capsulotomy has been the development of new IOL designs specifically for use with this technique.
These problems can lead—especially with use of premium IOLs—to unplanned refractive results, visual disturbances, and unhappy patients. Even worse, for example in the event of capsular phimosis or vitreous loss, higher rates of complications or the need for secondary surgery may result.
Like our own clinical results, studies by a number of authors have found that femtosecond laser capsulotomy is superior to the traditional CCC, a structure that has not changed much since its introduction by Gimbel and Neuhann in 1984.7-10
With the platform that we use (Lensar Laser System; Lensar), the localization of the capsulotomy is delivered as planned, centered on the pupil or the optical axis (Figure 5). In all patients the shape is a perfect circle, and the diameter is within ±0.1 mm of the planned size. In our own clinical experience, as in the literature,11,12 we achieve nearly 99% free-floating capsules without adhesions.
The literature also shows significantly better refractive outcomes and lower induction of higher-order aberrations in patients after laser capsulotomy compared with manual CCC,13,14 although we have yet to see whether these differences make a great clinical impact. Better contrast sensitivity with the laser seems to be another benefit,15-17 but, in order to assess this possible advantage, long-term studies will be necessary to demonstrate functional improvements for patients in daily life.
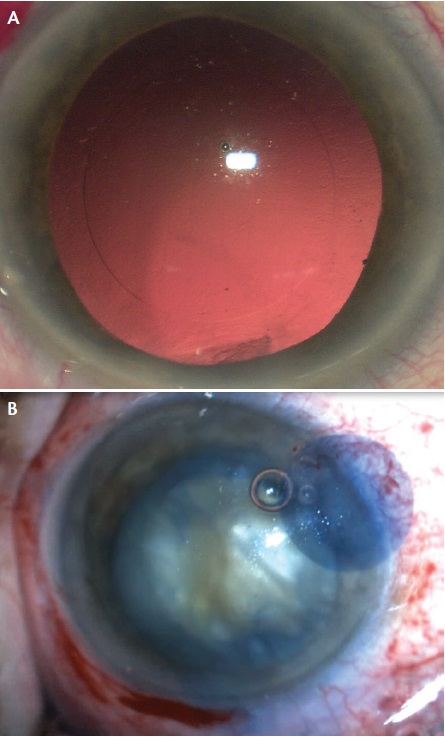
Figure 5. A perfect laser-assisted capsulorrhexis without (A) and with (B) the assistance of trypan blue dye.
SURGICAL PLANNING, EXECUTION CAN ENHANCE CAPSULOTOMY QUALITY
Consider the IOL. To obtain the best results with laser capsulotomy, surgical planning is crucial. The type of IOL to be implanted must be considered before deciding on the optimal size for the capsulotomy. For example, for a hydrophilic toric IOL with C-loop haptics, I prefer a relatively small diameter of 4.75 mm. The smaller diameter helps to reduce the possibility of rotation of the toric IOL due to the greater amount of capsule overlap of the IOL. By contrast, for a trifocal plate-haptic IOL such as the AT.LISA tri (Carl Zeiss Meditec), I prefer a larger diameter (5.75 mm) to facilitate better visual function in wider pupils and to reduce the risk of capsular phimosis.
Laser settings. The surgeon should review all laser settings in advance of surgery and adjust if needed. The laser energy, frequency of the laser shots, line spacing in the z-axis, and shot spacing all can be adjusted. The line spacing adjusts the height of the treated zone above and below the anterior capsule, and the shot spacing defines the distance between the individual laser spots in the x-y plane of the capsule. We prefer an energy level of 4 µJ, pulse repetition frequency of 80 KHz, line spacing in the z-axis of 15 µm, and shot spacing of 4 µm. With these parameters, we achieve excellent results regarding edge shape, free-floating capsules, and minimal thermal effect in the lens cortex.
Docking. The docking process is another surgical factor that may interfere with the quality of the capsulotomy. If the cornea shows distortions or the epithelium shows edema due to preoperative dryness, this may result in changes in the laser energy and incomplete cutting. The Lensar system has a liquid optics interface that inhibits corneal distortion because the cornea is not applanated as in other LACS platforms.
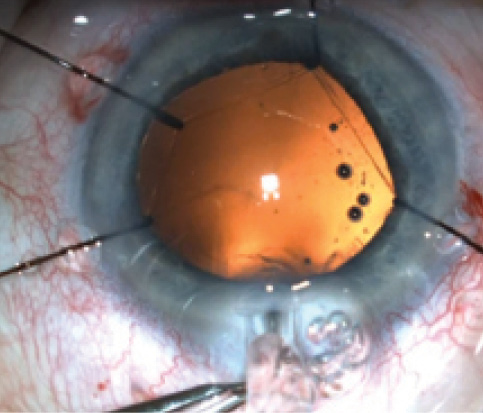
Figure 6. A femtosecond capsulorrhexis is resistant to stretching and tearing.
Imaging. The Scheimpflug imaging incorporated into our laser system is capable of detecting important anatomic landmarks such as the anterior capsule in almost every eye. Only in pupils of less than 4 mm is the system unable to perform a capsulotomy. Because we use Mydriasert (tropicamide 0.28 mg, phenylephrine HCl 5.4 mg; Thea Pharmaceuticals), this is an extremely rare occurrence. In the event of an undersized pupil, the use of pupil expansion rings is an option.
Execution. During the laser treatment, the surgeon must focus on the performance of the system and the progress of the laser capsulotomy. The duration of the capsulotomy treatment is approximately 2 seconds. During this time, in almost every case, the surgeon will see 360° of gas bubbles. If there is an area where the bubbles are less dense, this may be a sign of an incomplete cut. Conversely, more bubbles in an area of the capsule may also be a sign of an incomplete cut. This is important information to retain for subsequent removal of the capsule. Later, in surgery, the surgeon can take a closer look at these suspect areas of the capsule under the microscope.
I always use the dimple-down technique with an OVD and forceps to remove the capsule. Since I started using this technique, no capsular tears have occurred, and I always have a good view of the capsule. Possible adhesions are easy to notice, and, if necessary, a manual capsulorrhexis technique with forceps can be used to remove the capsule in the areas of adhesions.
RESULTS AND CASE REPORTS
In a prospective study of our own results, all patients exhibited a perfectly sized capsulotomy and better overlapping of the IOL in comparison with a control group with manual capsulorrhexis; the capsule was free-floating in 99% of eyes.11 Therefore, our data correspond well with the increasing number of other reports in the literature. As illustrated in Figure 6, these laser capsulotomies are highly resistant to tearing and shearing forces.
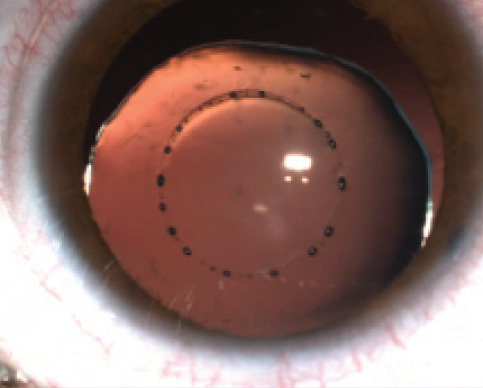
Figure 7. Laser-assisted capsulorrhexis in a Marfan patient with a subluxated lens.
In extreme cases, such as advanced white or brown cataracts or dislocated lenses as in Marfan syndrome, we can see another big advantage of femtosecond laser capsulotomy. In these types of difficult cataract cases, the risk of a capsular tear, dropped nucleus, and loss of vitreous is increased during manual capsulorrhexis. We have performed laser capsulotomy in white and brown cataracts without encountering problems. The percentage of adhesions in these eyes is higher than in standard cataract patients; however, with the use of a vital dye to stain the capsule intraoperatively, the capsule is always easily visualized, and its removal is safe.
We performed the first laser capsulotomy in a patient with Marfan syndrome with the Lensar system worldwide. The big advantage of the laser in this type of eye is the absence of stress on the zonula in comparison with manual capsulorrhexis. We have performed laser capsulotomy in several eyes in Marfan patients without intraoperative complications (Figure 7).
DEVELOPMENTS
One interesting side effect of the introduction of laser capsulotomy has been the development of new IOL designs specifically for use with this technique. In January 2014, we implanted the first LACS-specific lens in our clinic. The Femtis (Oculentis) is a hydrophilic, plate-haptic IOL that has four additional haptics that are enclaved in the capsulotomy. This leads to perfect centration in the capsular opening and minimal overlap of the capsule on the optic.
The idea of this concept is to optimize centration and to reduce the possibility of tilt, phimosis, and IOL rotation. Intraoperatively, there is a short learning curve, but we have experienced no IOL–design-related complications to date in more than 90 implantations. An ongoing multicenter study in which we are participating will assess the long-term performance of this new IOL.
For now, we can conclude that laser capsulotomy is a safe and precise procedure that decreases potential risks in cataract surgery. We recommend its use with premium IOLs and in difficult cases. In combination with specially designed IOLs, this brings cataract and refractive surgery into a new era. n
1. Ravalico G, Tognetto D, Palomba M, et al.Capsulorhexis size and posterior capsule opacification. J Cataract Refract Surg. 1996;22:98-103.
2. Aykan U, Bilge AH, Karadayi K. The effect of capsulorhexis size on development of posterior capsule opacification: small (4.5 to 5.0mm) versus large (6.0 to 7.0mm). Eur J Ophthalmol. 2003;13:541-545.
3. Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorhexis size on posterior capsular opacification:one-year results of a randomized prospective trial. Am J Ophthalmol. 1999;128:271-279.
4. Altmann GE, Nichamin LD, Lane SS, Pepose JS. Optical performance of 3 intraocular lens designs in the presence of decentration. J Cataract Refract Surg.2005;31:574-585.
5. Sugimoto Y, Takayanagi K, Tsuzuki S, et al. Postoperative changes over time in size of anterior capsulorhexis in phacoemulsification/ aspiration. Jpn J Ophthalmol. 1998;42:495-498.
6. Joo CK, Shin Ja, Kim JH. Capsular opening contraction after continuous curvilinear capsulorhexis and intraocular lens implantation. J Cataract Refract Surg.1996;22:585-590.
7. Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16:31-37.
8. Nagy ZZ, Kránitz K, Takacs AI, et al. Comparison of intraocular lens decentration parameters after femtosecond laser in cataract surgery. J Refract Surg. 2011;27(8):564-569.
9. Friedman NJ, Palanker DV, Schuele G, et al. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011;37(7):1189-1198.
10. Kránitz K, Takacs A, Miháltz K, et al. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011;27(8):558-563.
11. Holland D. Clinical results in cataract surgery using the Lensar femto cataract laser at the Eye Hospital Bellevue in Kiel. Paper presented at: the 18th Annual ESCRS Winter Meeting; February 14-16, 2014; Ljubljana, Slovenia.
12. Packer M, Teuma EV, Glasser A, Bott S. Defining the ideal femtosecond laser capsulotomy. Br J Ophthalmol. 2015;99(8):1137-1142.
13. Filkorn T, Kovács I, Takács A, et al. Comparison of IOL power calculation and refractive outcomes after laser refractive cataract surgery with a femtosecond laser versus conventional phacoemulsification. J Refract Surg. 2012;28(8):540-544.
14. Chee SP, Yang Y, Ti SE. Clinical outcomes in the first two years of femtosecond laser-assisted cataract surgery. Am J Ophthalmol. 2015;159(4):714-719.e2.
15. Kránitz K, Miháltz K, Sándor GL, et al. Intraocular lens tilt and decentration measured by scheimpflug camera following manual or femtosecond laser created continuous circular capsulotomy. J Refract Surg. 2012;28(4):259-263.
16. Miháltz K, Knorz MC, Alió JL, et al. Internal aberrations and optical quality after femtosecond laser anterior captulotomy in cataract surgery. J Refract Surg. 2011;27(10):711-716.
17. Alio JL, Abdou AA, Puente AA, et al. Femtosecond laser cataract surgery: updates on technologies and outcomes. J Refract Surg. 2014;30(6);420-427.
Richard R. Schulze Jr, MPhil (Oxon), MD
• Private Practice, Schulze Eye & Surgery Center, Savannah, Georgia
• richardschulze@comcast.net
• Financial disclosure: None
Detlef Holland, MD
• Augenklinik Bellevue, Kiel, Germany
• d.holland@augenklinik-bellevue.de
• Financial disclosure: Consultant (Lensar)
