

The use of a femtosecond laser to perform certain aspects of cataract surgery was first reported in 2009.1 More recently, intraoperative image-guidance systems for use in conjunction with the laser have become available.2 These technologies enable cataract surgeons to perform clear corneal incisions (CCIs), limbal relaxing incisions (LRIs), capsulotomy, and nucleus fragmentation with precision.
AT A GLANCE
• In the authors’ study, FLAAK was safely performed in post-PKP patients after all sutures were removed and at least 3 months after the patient was deemed reasonably keratometrically and refractively stable.
• In order to get the FLAAK incisions centered in the graft, the surgeon can manually move the blue line on the laser scan, which designates the limbus, until the incisions are centered exactly as desired.
• With intraoperative imaging, the surgeon can see an effect of the FLAAK incisions right away, on the table, although this does not reflect the final outcome.
The principal use of these laser and image-guidance technologies to date has been in refractive cataract surgery; however, we felt that they could also be used to perform astigmatic keratotomy (AK), potentially providing more precise and predictable results than manual AK for the management of astigmatism in a variety of situations. For example, femtosecond laser-assisted AK (FLAAK) could be used for enhancements in pseudophakes who might not be ideal candidates for excimer laser vision correction.
Soon after we acquired the Catalys Precision Laser System (Abbott Medical Optics), we began to use the machine off-label to perform FLAAK enhancements in pseudophakes. Selected patients had a spherical equivalent refraction of near plano with astigmatism that we considered to be amenable to correction with AK. We had great results with FLAAK in these patients and presented data on the results at several meetings.3,4
This mode of correction is easy for the patient. Visual recovery is almost immediate, there is little down time, and it provides a reasonably predictable outcome.
POSTKERATOPLASTY FLAAK
Based on our encouraging experience with FLAAK in pseudophakes, we decided to explore the use of the same technique and technology for other indications: specifically, for residual astigmatism after PKP.
We now have 1-year follow-up for FLAAK in a series of 11 patients with high astigmatism after PKP. The laser procedure was performed after all sutures were removed and at least 3 months after the patient was deemed reasonably keratometrically and refractively stable. We plan to present the 1-year follow-up results in these patients at the 2016 American Society of Cataract and Refractive Surgery (ASCRS) meeting.3 The remainder of this article briefly describes our technique and the special considerations for performing FLAAK in patients after PKP.
DIFFERENCES between normal, POST-PKP eyes
There are two key differences between normal and post-PKP eyes in regard to astigmatism correction.
Architecture of astigmatism. After PKP, the eye’s architecture of astigmatism is quite different from that of regular corneal astigmatism in a normal eye. The donor-recipient interface creates an artificial limbus, which is roughly 8 mm in diameter. Additionally, the centration of this new limbus is not perfect, as the manually performed corneal graft is never precisely in the center of the cornea.
Variable wound healing and scar contracture. At the new limbal interface in the post-PKP eye, both wound healing and scar contracture are variable. These two forces can induce high levels of irregular astigmatism, and the mismatch of donor and recipient tissues can also create high levels of astigmatism. Particularly in patients who have had PKP because of corneal scars, corneal ulcers, or stromal scars, both contracture and wound healing can cause high levels of astigmatism.
For these reasons, routine nomograms for AK do not apply to post-PKP patients. Therefore, in these cases, any astigmatism reduction should be based completely on a careful physical examination, including corneal tomography. The physical examination should assess for the presence of wound contraction or wound dehiscence; an AK incision in an area of wound contraction can generate a large refractive effect. Corneal tomography that documents both the anterior and posterior surfaces of the cornea, such as the Pentacam (Oculus) or Galilei (Ziemer), is necessary to rule out graft ectasia. We also assess mesopic and photopic pupil size in the preoperative examination. We do not want the incisions to be placed in the visual axis, as glare and halos can result. During surgical planning, we then make sure the incisions will be outside of the mesopic pupil.
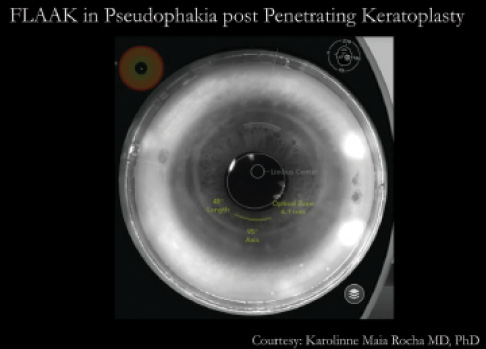
Figure 1. In post-PKP eyes, the artificial limbus can be adjusted to achieve proper centration within the graft.
PLANNING, SETTINGS
If the patient has regular astigmatism with an identifiable axis, in the best-case scenario, the keratometry will match the manifest refraction, and that axis can be used for the laser incision. For an asymmetric bowtie, an asymmetric chord length can be used, with the larger chord on the larger hemimeridian of the asymmetric bowtie.
Incisions are made at 80% depth inside the graft. For FLAAK in a normal eye, the optical zone would be 9 mm. In post-LASIK eyes, the optical zone is increased by 1 mm, to 10 mm, in order to cut outside the flap. In post-PKP patients, however, we want to stay inside the graft. Because corneal grafts average about 8 mm in diameter, the AK optical zone is usually between 6 and 7 mm, an average of about 6.5 mm.
The nice thing about the Catalys software is that the incisions can be moved in and out manually on the touchscreen in real time. The scan of the eye appears on the screen, and the surgeon can simply use a finger to position the incisions as desired.
Because of the small optical zone, we do not recommend using AK incisions of greater than 45° arc length. With the smaller optical zone, these incisions have more effect than they would at a 9- or 10-mm zone, especially if they are placed in an area of scarring. Also, if too much effect is achieved, ectasia can develop in the graft. This is why, preoperatively, we must rule out areas of thinning or bulging, as in keratoconus or postrefractive surgery ectasia.
With intraoperative imaging, the surgeon can see an effect of the incisions right away, on the table, although this does not reflect the final outcome. Because of the biomechanical effects of the healing process, we wait at least 6 months before doing any enhancement. If a greater effect is needed, at the 1-month follow-up visit, a Sinskey hook can be used to open the incision.
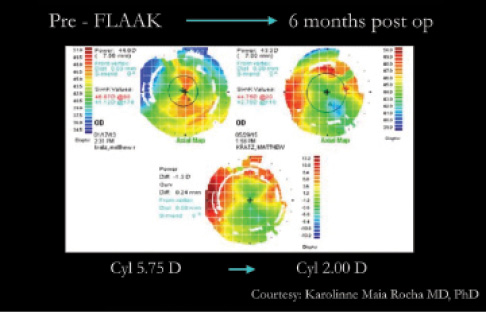
Figure 2. In a post-PKP eye with 5.75 D cylinder, FLAAK reduced the cylinder to 2.00 D at 6 months postoperative.
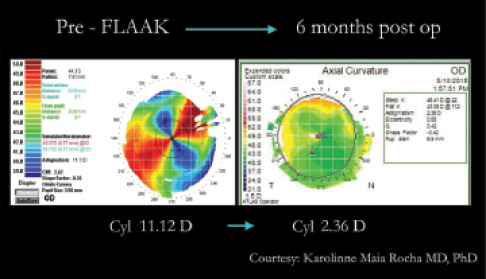
Figure 3. In a post-PKP eye with 11.12 D cylinder, FLAAK reduced the cylinder to 2.36 D at 6 months postoperative.
TRICKING THE LASER
Proper centration of the incisions is important in post-PKP eyes. As noted above, transplant grafts are never exactly in the center of the cornea. They may be displaced nasally, temporally, superiorly, inferiorly, or some combination. In order to achieve proper centration of the incisions in the graft, it is often necessary to trick the laser software, so to speak. For normal eyes, the software is programmed to center AK incisions in relation to the limbus, but, in a post-PKP eye, this means the incisions would be decentered within the graft. On the laser scan of the eye, the limbus is indicated with a blue line. In order to get the AK incisions centered in the graft, the surgeon can manually move that blue line until the incisions are exactly centered as desired (Figure 1).
Another trick is needed when FLAAK is done in pseudophakic patients, as were all the cases in our series. The laser’s safety recognition mechanisms are calibrated to recognize the human crystalline lens, so the laser will not proceed in pseudophakic patients because it cannot recognize the crystalline lens. To defeat this, the user can manually override the lens capture software, allowing the laser to go forward with the treatment. Additionally, the other features normally used in cataract surgery—creation of the CCI and capsulotomy and softening of the lens—can be overridden, and only the LRI components utilized.
CONCLUSION
In post-PKP patients, who tend to have substantial and irregular astigmatism, FLAAK can have a significant benefit for postoperative outcomes. With a reasonable standard deviation, we have achieved positive results using this minimally invasive procedure in a series of 11 post-PKP patients. In all cases, the procedure greatly enhanced quality of life, and results steadily improved up to 6 months and then remained stable from 6 to 12 months. There is a reasonable amount of fluctuation after the procedure, so enhancements are not undertaken for a prolonged period postoperatively. Generally, however, this fluctuation is within an overall greatly reduced magnitude of astigmatism, with improvements seen in refraction, keratometry, tomography, visual acuity, and patient satisfaction (Figures 2 and 3).
We believe that FLAAK improves the precision and predictability of AK just as laser-assisted cataract surgery has improved the precision and accuracy of astigmatism control in refractive cataract surgery. We believe that there are a number of applications in which patients can truly benefit from FLAAK, including enhancement of astigmatic results in pseudophakes and after PKP.
1. Nagy Z, Takacs A, Filkorn T, et al. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery.
J Refract Surg. 2009;25:1053-1060.
2. Batlle JF, Feliz R, Culbertson WW. OCT-guided femtosecond laser cataract surgery: precision and efficacy. Poster presented at: Association for Research in Vision and Ophthalmology Annual Meeting. May 1-5, 2011; Fort Lauderdale, Florida.
3. Koike K, Waring IV GO, Rocha KM. Femtosecond laser-assisted arcuate keratotomy for treatment of astigmatism after penetrating keratoplasty. Paper presented at: the American Society of Cataract and Refractive Surgery meeting; May 6-10, 2016; New Orleans, Louisiana.
4. Waring IV GO. Femtosecond laser-assisted astigmatic keratotomy for pseudophakic enhancements. Paper presented at: the American European Congress of Ophthalmic Surgery Winter Congress; Aspen, Colorado; February 26, 2014.
Karolinne M. Rocha, MD, PhD
• Director of Cornea Service and Assistant Professor, Storm Eye Institute, Medical University of South Carolina
• karolinnemaia@gmail.com
• Financial disclosure: Consultant (Abbott Medical Optics)
George O. Waring IV, MD, FACS
• Director of Refractive Surgery and Associate Professor of Ophthalmology, Storm Eye Institute, Medical University of South Carolina
• Medical Director, Magill Vision Center, Mount Pleasant, South Carolina
• waringg@musc.edu
• Financial disclosure: Consultant (Abbott Medical Optics)
