
Over the years, pterygium excision techniques have evolved, from the crude bare sclera technique that had been used since medieval times to modern sutureless techniques that result in excellent cosmetic results with minimal patient discomfort. Today, the most commonly performed technique, pterygium excision with a conjunctival autograft to cover the excised tissue bed, has a recurrence rate of 5%.1 In one recent report, a pterygium excision technique involving a combination of conjunctival autograft and subconjunctival amniotic membrane graft had a recurrence rate of 1% in a low-risk patient population.2
AT A GLANCE
- In a recent report, a pterygium excision technique involving a combination of conjunctival autograft and subconjunctival amniotic membrane graft had a recurrence rate of 1% in a low-risk patient population, compared with a 5% recurrence rate with the traditional technique.
- Among the indications for surgical treatment with the technique are growth of the lesion over time, reduction in visual acuity, recalcitrance to topical treatments using lubrication and antiinflammatory drops, chronic recurrent inflammation, cosmetic concerns, and the inability of the patient to wear contact lenses due to the size or location of a lesion.
- The technique includes prophylactic placement of subconjunctival amniotic membrane, which requires a moderate degree of surgical skill beyond that of a conjunctival autograft. Nevertheless, this technique deserves consideration for all primary pterygium procedures and especially for high-risk cases in which traditional pterygium treatments are more likely to fail.
A colleague and I recently reported results in a retrospective study in which a similar technique was used, over a period of 7 years, in 493 eyes of 355 patients with clinically significant pterygia that warranted surgical excision.3 (For a description of the procedure, see Surgical Technique below.) This was a population with a high potential for recurrence (Latino, Afro-Caribbean, and Asian individuals), but, using a combination of conjunctival stem-cell grafting and amniotic membrane, a low recurrence rate was observed (1.22%).
SURGICAL TECHNIQUE
The eye is anesthetized with injection of subconjunctival bupivacaine 0.025% to balloon the area of the pterygium and fibrotic Tenon tissue. The pterygium is then excised with Wescott scissors and toothed forceps, with an effort to retain as much normal-appearing conjunctiva as possible (without actinic changes). I use toothed forceps and blunt Wescott scissors to dissect the pterygium fully from the underlying sclera up to the corneoscleral limbus, then avulse the pterygium by stabilizing the eye with a dry cotton-tip applicator while applying gentle traction to the pterygium with the toothed forceps.
I sharply dissect the fibrovascular Tenon tissue from the overlying conjunctiva back toward the medial rectus muscle, and I use wet-field cautery as necessary for hemostasis. A rotating diamond burr is used on bare sclera and the corneal limbus to strip any remaining adherent fibrovascular tissue, creating a smooth bed. After this dissection, a subconjunctival space is available for later placement of amniotic membrane tissue with freely mobile overlying conjunctiva.
I carefully measure the smooth scleral bed to determine the size of the conjunctival autograft, which will be oversized by 1 mm in horizontal and vertical dimensions to ensure complete coverage of the bed. The autograft is harvested from the superior conjunctiva, which has had minimal exposure to UV light. I make every effort to create a thin graft by dissecting Tenon fascia from the underside. Using a diamond blade, I superficially score the corneal epithelium 1 mm anterior to the limbal vascular arcades to obtain limbal stem cells, as this tissue helps inhibit recurrence. I carefully mark the area to maintain orientation when gluing the graft in place.
Dehydrated amniotic membrane tissue is cut into a strip, the size of which depends on the size of the bed. I wet this strip with a small volume of fibrin sealant (Evicel; Omrix Biopharmaceuticals). I then introduce the membrane into the previously created subconjunctival space. The amniotic membrane that remains in the subconjunctival space will be covered by the conjunctival graft.
I dissect the conjunctival graft free from the superior limbus and leave it inverted on the cornea so that the epithelium of the graft is facing the epithelium of the cornea. I then move the graft to the pterygium excision site, maintaining its orientation so that the limbal side of the graft corresponds to the limbal side of the excision site. I place thrombin on the scleral bed of the excision site and fibrinogen on the Tenon fascia side of the graft.
Using nontoothed forceps, I invert the graft so that the thrombin and fibrinogen are mixed on the scleral bed, and I gently stretch the graft to cover the exposed sclera using nontoothed forceps. I remove excess sealant with a clean cotton-tip applicator and trim excess graft tissue where necessary. A bandage contact lens is placed to help keep the graft flat at the limbus and to provide comfort by covering the corneal epithelial defect that resulted from the pterygium avulsion.
Postoperatively, the eye is gently taped shut for an hour. Medication includes topical moxifloxacin 0.5% (Vigamox; Alcon) and bromfenac 0.09% (Bromsite; Sun Pharmaceuticals) for the first week, at which time the bandage contact lens is removed. The patient also uses lotepredenol etabonate ophthalmic gel 0.5% (Lotemax; Bausch + Lomb) four times daily for the first 2 weeks, three times daily for the second week, twice daily for the third week, and once daily after that until the conjunctival graft is quiet.
I see patients for a postoperative visit at day 1, then within 1 week after surgery (Figure 1) and at 1 month (Figure 2). The bandage contact lens is removed at the 1-week visit. I examine the eye again at the slit lamp at least 6 months after surgery for signs of recurrence or complications.
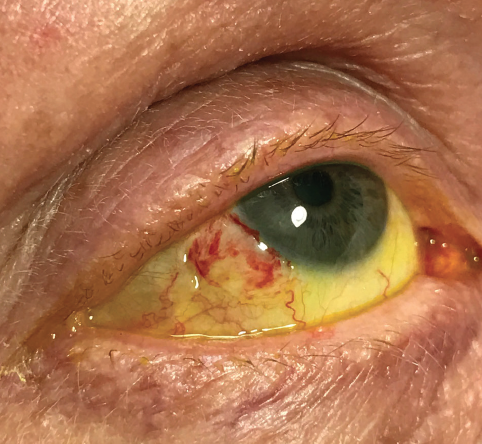
Figure 1. One week after surgery.
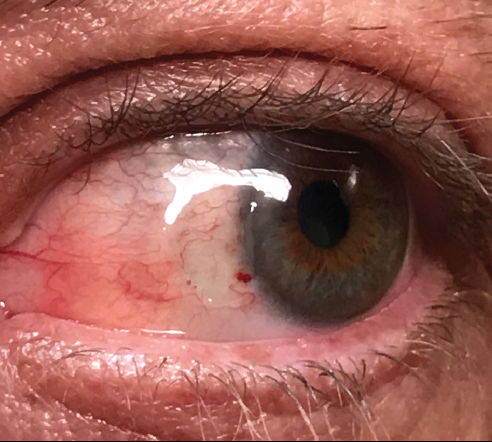
Figure 2. One month after surgery.
Based on this experience dealing with these and other patients, in this article I outline five important issues that can help to maximize outcomes in patients with clinically significant, actinic, ocular surface disease (OSD). Among the indications for surgical treatment are growth of the lesion over time, reduction in visual acuity, recalcitrance to topical treatments using lubrication and antiinflammatory drops, chronic recurrent inflammation, cosmetic concerns, and the inability of the patient to wear contact lenses due to the size or location of a lesion.

Carefully evaluate the ocular surface
Small pingueculae or pterygia may be overlooked by the clinician when patients present complaining of irritation, foreign body sensation, or visual disturbance. Carefully examine eyelid position (ectropion or entropion), look for lagophthalmos, and assess blink rate. These may be the etiology or just exacerbating symptoms if other pathology is present.
Staining with both fluorescein and a vital dye such as lissamine green, evaluating the precorneal tear film, and looking at tear breakup time may provide valuable clues. Inspection of the bulbar and palpebral conjunctiva may expose a pingueculum or pterygium that stains despite an initial subclinical appearance. Keratitis sicca, rosacea, or another OSD may magnify the severity of any actinic changes on the conjunctiva or cornea, and these conditions should be treated primarily. Sometimes, topical therapy with cyclosporine ophthalmic emulsion 0.05% (Restasis; Allergan) or lifitegrast ophthalmic solution 5% (Xiidra; Shire), pulsed topical steroids, and punctal occlusion may preclude the necessity for surgical intervention.

A pterygium often induces astigmatism
Often, a pterygium induces a significant effect on astigmatism. As pterygia tend to be found on the nasal aspect of the limbus, a significant flattening effect can be seen in the horizontal axis. This is easily visualized on topographic studies and usually corresponds to the patient’s refraction.
When pterygia are found in conjunction with visually significant cataracts, a staged procedure should be considered. I usually perform excision of the pterygium with a conjunctival stem-cell graft and amnion and allow a minimum of 6 weeks for the ocular surface to recover. In most cases, this reduces the amount of astigmatism before I proceed to take preoperative measurements for refractive or cataract surgical procedures.

Leave Tenon tissue to prevent graft retraction
Tenon fascia is a thin membrane beneath the conjunctiva and adherent to it. It envelops the globe from the optic nerve posteriorly to the limbus anteriorly and separates the globe from the orbital fat with delicate bands of connective tissue linking the fascia to the sclera.
When harvesting a free conjunctival graft, it is of utmost importance to make the graft as thin as possible, leaving behind Tenon tissue from the harvest site. This usually prevents graft retraction, which exposes sclera and increases the risk of recurrence.4
Oversizing the graft by 1 mm in both horizontal and vertical dimensions relative to the excised bed also reduces the chance of retraction and provides a better cosmetic result.

Include limbal stem cells in the graft
Inclusion of limbal stem cells in the conjunctival graft has been shown to hasten reepithelialization of the corneal defect and to reduce the likelihood of pterygium.1 When harvesting the graft, I score the corneal epithelium superficially 1 mm in front of the limbal arcades where these cells reside,5 and I mark the graft to maintain orientation. When dissecting the graft from the conjunctiva toward the limbus, I undermine using a crescent-shaped blade to include these cells in the transplanted tissue.

Preserved amniotic membrane can function as a basement membrane substitute or temporary graft
Amniotic membrane tissue has antiinflammatory and antiscarring effects. It contains growth factors that promote epithelial wound healing on the ocular surface. It has been found to be an option for corneal and conjunctival reconstruction in many clinical situations, including acute burns, persistent corneal epithelial defects, and diseases that cause scarring. It has gained widespread use since 1995 because of newer preservation techniques.6
I usually employ dehydrated amnion, cutting or folding it to size to fit the subconjunctival space at the distal end of the excised pterygium bed. It is presumed that recurrences arise from the Tenon tissue; therefore, the added benefit of amnion and the conjunctival graft with stem cells has been effective.
CONCLUSION
Specialists have considered the gold standard for primary pterygium treatment to be excision with a conjunctival autograft.7 Still, recurrences occur with this technique, especially in high-risk patients, including men, those exposed to excessive UV light, darkly pigmented people, and those under age 40 years.
Prophylactic placement of subconjunctival amniotic membrane requires a moderate degree of surgical skill beyond that of a conjunctival autograft. Nevertheless, this technique deserves consideration for all primary pterygium procedures and especially for high-risk cases in which traditional pterygium treatments are more likely to fail.
Based on the clinical experience related earlier, with a recurrence rate of 1.22% in a high-risk population, I believe that pterygium excision using conjunctival autografts with prophylactic placement of subconjunctival amniotic membrane will have a more desirable recurrence rate. Although further study will help to define exactly which patients are mostly likely to benefit from this technique, I believe that this combined technique deserves consideration.
1. Kenyon KR,Wagoner MD, and Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985;92:1461-1470.
2. Shusko A, Hovanesian JA. Pterygium excision with conjunctival autograft and subconjunctival amniotic membrane as antirecurrence agents. Can J Ophthalmol. 2016;51(6):412-416.
3. Shusko A, Schechter BA. Outcomes of pterygium excision with a conjunctival autograft and subconjunctival amniotic membrane graft as a prophylactic anti-recurrence agent in ethnically diverse populations. Paper presented at: The American Society of Cataract and Refractive Surgery Annual Meeting. May 5-9, 2017; Los Angeles, CA.
4. Figueira EC, Coroneo MT, Francis IC. Preventing conjunctival autograft inversion in pterygium surgery. J Ophthalmol. 2007; 91(1):83-84.
5. Schermer A, Galvin S, and Sun TT. Differentiation-related expression of a major 64k corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49-62.
6. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473-484.
7. Fernandes M, Sangwan VS, Bansal AK, et al. Outcome of pterygium surgery: analysis over 14 years. Eye (Lond). 2005;19(11):1182-1190.


