Dry eye disease (DED) is everywhere, and in fact it is the most frequently encountered disease state by eye care professionals. According to a 2013 Market Scope analysis,1 more than 25 million people living in Europe suffer from DED. What is an even more staggering statistic is that these people spend about €3.2 billion annually in the European Union on medications and prescriptions that provide DED symptom relief. According to Market Scope, the global dry eye treatment market will reach $4.9 billion in 2022, with the highest rate of growth expected in China, India, and other emerging markets.
Given these statistics, it is no wonder that we see so many patients with DED. The presence of DED is not a contraindication to refractive surgery, however, as long as it is recognized and treated adequately prior to scheduling surgery. In this article, I will review the two most primary forms of DED and overview some of the best methods to treat the condition.
CATEGORIZE THE DED
Before you can recognize the presence of DED, you must understand the difference between the two primary forms, evaporative and aqueous deficient.
Evaporative DED. This form of DED, which is a lipid deficiency caused by meibomian gland dysfunction, accounts for roughly 86% of the DED patients you will see.2 Evaporative DED occurs when the aqueous in tears evaporates at a faster than normal rate. The severity of the disease can range from mild to severe.
Aqueous deficient DED. This form of DED is more rare, as it only constitutes about 14% of the DED patients you will see.2 In patients with aqueous deficient DED, aqueous generation from the lacrimal gland is insufficient to keep the eyes moist. The severity of the deficiency can range from a tear meniscus dimension of 0.2 to 0.0 mm. Treatments to maintain or increase tear volume should be considered in patients with tear meniscus heigh values ≤210 µm because they have a 4.65 relative risk ratio of developing severe corneal epitheliai disease (odds ratio = 5.59).3
DIAGNOSE THE DED
Diagnosing both evaporative and aqueous deficient DEDs requires both subjective and objective testing. Luckily for us, the Tear Film and Ocular Surface Society recently released its Dry Eye Workshop II (DEWS II) study,4 which generated a global consensus on multiple aspects of DED, including an updated definition and classification system, a regimen for diagnosis of DED, and recommendations for pharmaceutical interventions for DED treatment (Figure 1).
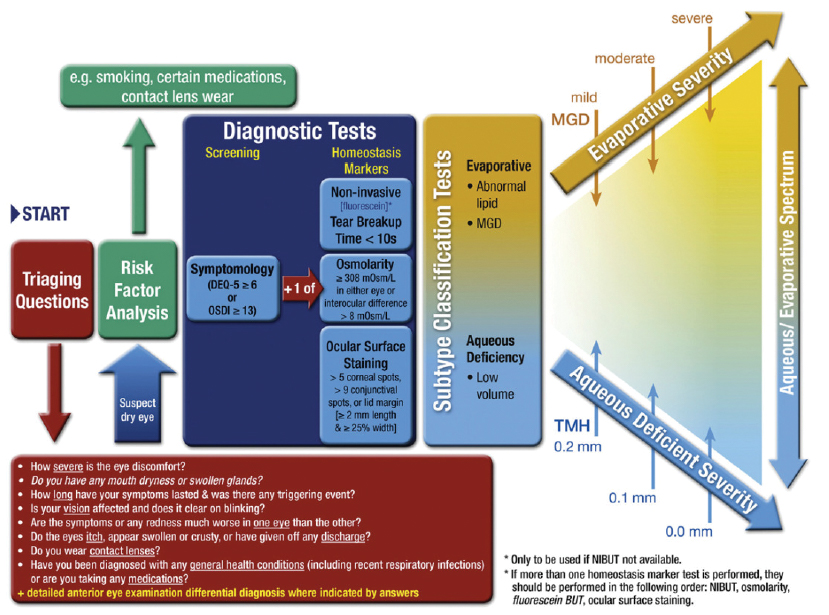
Figure 1. TFOS DEWS II flow chart.
The first and foremost step is to talk to each patient who walks through your door. A DED questionnaire such as the Standardized Patient Evaluation of Eye Dryness (SPEED) or Ocular Surface Disease Index (OSDI) are both appropriate and helpful tools to help identify patients with DED. Further, patients should be asked about what kind of medications they are on and have been on in the past, including antihistamines, decongestants, antidepressants, and glaucoma eye drops. Patients should also be assessed for any anatomical lid defects. Another important step in the analysis of patients for DED is to perform a systemic history, including Sjogren syndrome, rheumatoid arthritis, rosacea, thyroid dysfunction, lupus, and hormonal changes.
In my office, I perform the following diagnostic testing in addition to the SPEED/OSDI patient questionnaires:
- Corneal and conjunctival staining with fluorescein, lissamine green, or rose Bengal;
- Tear film breakup time (TBUT);
- Schirmer test;
- Meibography;
- Tear osmolarity;
- Matrix metallopeptidase 9 (MMP-9) and interleukin-1 receptor antagonist; and
- Epithelial thickness mapping.
A DEEPER DIVE
Some of the diagnostic testing we have available today is more advanced than the staining, TBUT, and Schirmer tests that most of us are comfortable performing. These tests are still extremely relevant and important to perform, but other tests can help us to pinpoint the exact cause and severity of the DED.
Tear osmolarity. This is a relatively new innovation that can be useful to detect abnormal osmolarity or instability in the tear film. Normal tear osmolarity is 290 mOsms/L, but patients with DED will often present with an elevated reading (>308 mOsms/L), indicating loss of homeostasis. Likewise, the typical inter-eye difference in tear osmloarity is 2 mOsm/L; however, patients with DED who present with a difference of >8 mOsms/L have instability of the tear film. If the patient is symptomatic but presents with normal tear osmolarity, additional considerations include conjunctival chalasis, mild allergic conjunctivitis, and epithelial basement membrane dystrophy.
It should also be noted that patients who do not present with DED symptoms can still have hyperosmolarity, and performing a tear osmolarity test will help to identify such patients. According to a study of 9,216 patients performed by Sullivan et al,5 51% of patients had a normal osmolarity, meaning that the other 49% had some form of DED. Of the patients who did not report any symptoms of DED (n = 5,191), 47% had hyperosmolarity.
Tear film assessment. TBUT can be assessed thoroughly with the HD Analyzer (Visiometrics). This tool can not only differentiate between eyes with healthy and unhealthy tear films, but it can also differentiate between a healthy eye with a normal TBUT cycle and a healthy eye with an accelerated TBUT cycle (Figure 2). The HD Analyzer can also create an ocular scattering index, which can correlate with visual function.
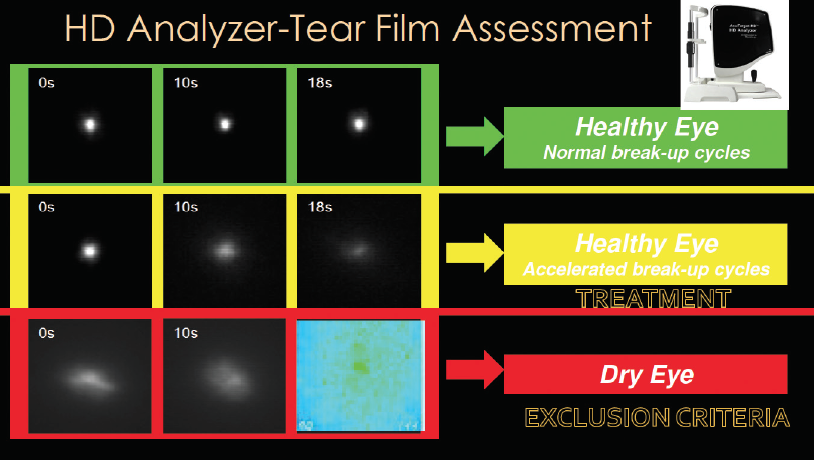
Figure 2. Tear film assessment with the HD Analyzer.
Inflammation. As another indicator of DED, it is important that we test the ocular surface for inflammation. Identifying elevated MMP-9 levels guides our therapeutic decision making because it can help to predict what patients will respond to antiinflammatory therapy, and it can therefore help us to customize a treatment plan.6 Traditional testing methods (TBUT, Schirmer, and even osmolarity) cannot predict what patients have inflammation, and therefore I use the RPS Inflammadry test (RPS).
In the presence of inflammation, many complications can occur if cataract or refractive surgery is attempted. These include less accurate presurgical measurements, leading to potentially worse visual acuity outcomes,7 and exacerbation of DED severity and symptoms.8
PUTTING IT ALL TOGETHER
So why does diagnosing (Figure 3) and treating DED preoperatively matter? For starters, it will help you to ensure that your patients have the best shot at excellent postoperative outcomes (Figure 4). Specifically, leaving a patient hyperosmolaric prior to IOL implantation could result in a significant difference in IOL power, as indicated by Epitropoulos et al.9
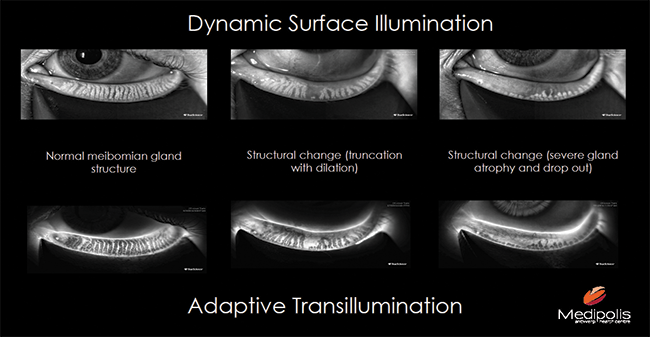
Figure 3. Dynamic surface illumination and adaptive transillumination help to assess the Meibomian gland structures.
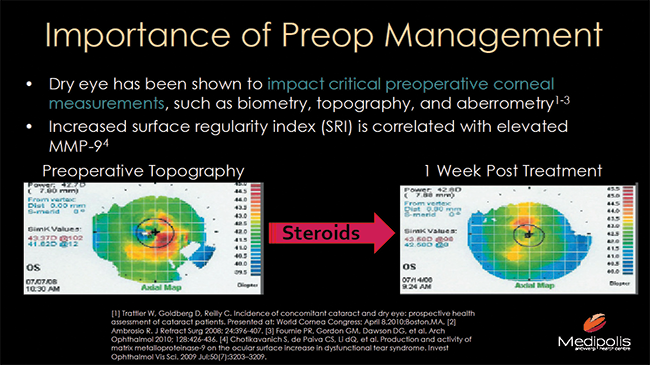
Figure 4. Depiction of how DED can impact preoperative corneal measurements: Preoperative topography (left) and repeat topography 1 week after prescribing treatment with steriods (right).
1. Market Scope. 2013 Comprehensive Report on the Global Dry Eye Products Market. St. Louis, Mo: Market Scope, November 2013.
2. Lemp MA, et al. Distribution of aqueous deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea. 2012;31(5):472-478.
3. Tung CI, Perin AF, Gumus K, Pflugfelder SC. Tear meniscus dimensions in tear dysfunction and their correlation with clinical parameters. Am J Ophthalmol. 2014;157(2):301-310.e1.
4. Jones L, Downie LE, Korb D, et al. TFOS DEWSII management and therapy report. Ocul Surf. 2017;15(3):575-628.
5. Sullivan BD, Crews LA, Messmer EM, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: Clinical implications. Acta Ophthalmologica. 2012. doi:10.1111/aos.12012.
6. Kaufman HE. The practical detection of MMP-9 diagnoses ocular surface disease and may help prevent its complications. Cornea. 2013;32:211-216.
7. Trattler W, Goldberg D, Reilly C. Incidence of concomitant cataract and dry eye: Prospective health assessment of cataract patients. Presented at: World Cornea Congress; April 8, 2010; Boston.
8. Ambrosio R. J Refract Surg. 2008;24:396-407.
9. Epitropoulos AT, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41:1672-1677.

