The goal of CXL therapy is not only to strengthen the cornea to slow or stop the progression of keratoconus, but also to induce apex flattening and improve corneal symmetry, which can lead to refractive improvement.
Conventional accelerated CXL protocols demonstrate a medium- to long-term improvement in visual acuity and topographic and aberrometric parameters,1,2 but the results are highly variable because of the uneven collagen biological response to photodynamic reaction. The goal of this article is to guide surgeons and help them choose the best approach: a single CXL procedure or CXL in combination with transepithelial PRK (CXL-plus) to stabilize keratoconus and reduce aberrations, especially coma. In this regard, we will discuss the selective transepithelial ablation for regularization of ectasia (STARE-X) protocol for treating corneal ectatic disorders.
Fundamental 1: Therapeutic strategy must be influenced by the patient’s history, behavior, and age
CXL is strongly suggested for patients under age 18 years who are diagnosed with keratoconus, rub their eyes excessively, and have a history of ocular allergy.3,4 CXL alone is an effective option for patients with BCVA better than 20/40 and a low degree of comatic aberrations because the goal is to halt keratoconus progression while the patient still has good visual acuity. Treating ocular allergy and encouraging patients to stop eye rubbing will also help to reduce external factors that are actively involved in keratoconus progression (see Case Report).
Case Report
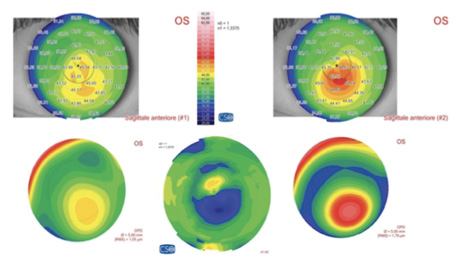
Figure 1. Preoperative sagittal and aberration maps (top and bottom right), postoperative sagittal and aberration maps (top and bottom left), and differential curvature map (bottom center) of a patient with keratoconus, thin corneas, and cone apex of 50.00 D treated with decentered corneal wavefront-guided transepithelial PRK plus accelerated CXL. A marked corneal flattening in the ectatic area was observed, associated with a strong reduction of comatic aberration.
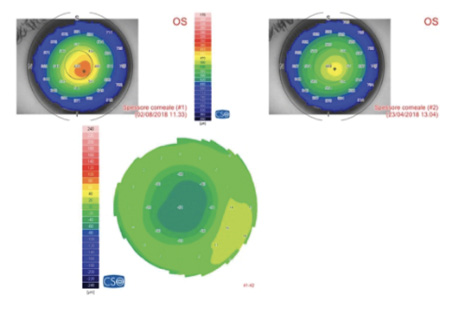
Figure 2. Pachymetric map of the patient in Figure 1. The differential map (bottom) shows corneal thinning of 60 µm in the apex zone 6 months after laser epithelium removal plus accelerated CXL.
Fundamental 2: Increase the safety of epithelium-off CXL using an accelerated protocol and customizing dosage and energy to the patient’s corneal pachymetry, especially in thinner corneas
Accelerated, customized CXL treatment can be performed safely in normal and thin corneas (< 400 μm) with different UV-A power and exposure time. The use of hydroxypropyl methylcellulose riboflavin solution is mandatory when managing thin corneas to help prevent corneal dehydration induced by dextran.5-8 The nomogram in Figure 1 shows the depth of the demarcation line after CXL is performed with different energy levels and a fixed UV-A dose of 5.4 J.9
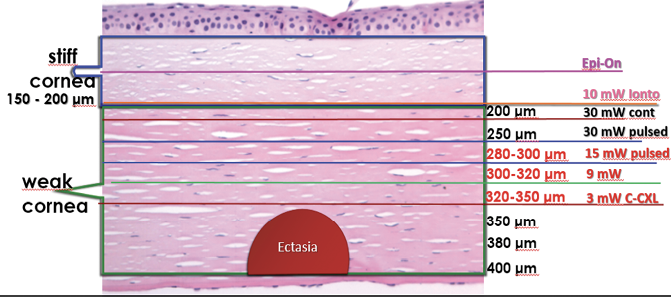
Figure 1. The depth of the demarcation line after CXL with different energy levels and a fixed UV-A dose of 5.4 J.
Our advice to improve the safety of this treatment is to consider a CXL protocol that will produce a demarcation line at least 100 μm away from the endothelium. For this reason, it is better to avoid the Dresden protocol (3 mW/cm2, 5.4 J energy) in corneas thinner than 400 μm (Figure 1).
Fundamental 3: In early-stage keratoconus, consider the difference between central and peripheral cones before planning treatment, especially when using an old CXL machine
Several studies have shown that cone location is one of the most important parameters involved in corneal flattening after CXL performed with the Dresden protocol. Greenstain et al9 demonstrated that, after CXL, more topographic flattening occurs in eyes with centrally located cones, and the least flattening effect of the cone occurs when the cone is located peripherally.10 This cone-location effect is found in eyes with both keratoconus and ectasia with an average difference of more than 2.00 D.
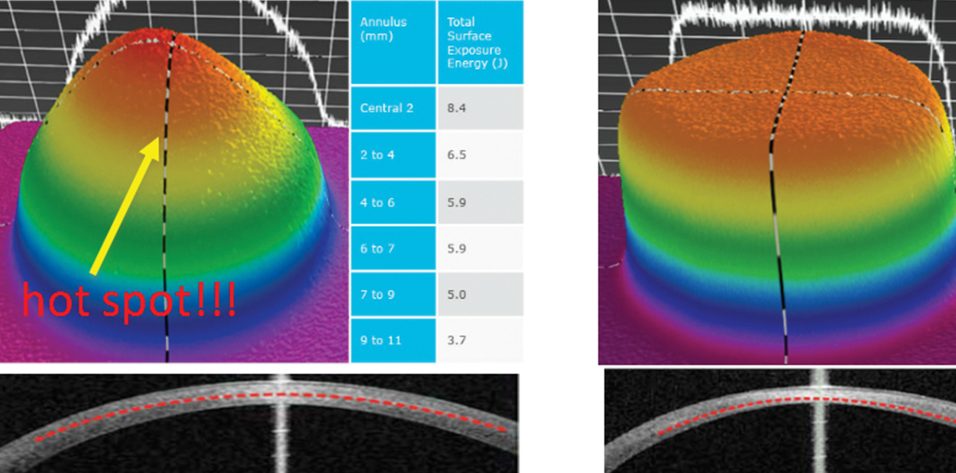
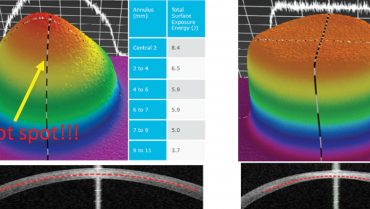
The flat irradiation beam delivered by older CXL machines does not work well with the curvilinear shape of the cornea. Studies examining energy absorption profiles demonstrated a significant difference (> 4 J) between the central and peripheral cornea, creating an uneven energy delivery profile (Figure 2).11 If a flat beam profile irradiates a convex surface (ie, the cornea), the central area will absorb more energy because it is closer to the energy source compared with the periphery. The other issue is that, in eyes with keratoconus, the location of the cone apex is typically in the paracentral and peripheral cornea (2 mm from the corneal center). The result is that a CXL procedure performed with an older machine centered on the cornea will produce more crosslinking (and flattening) effect on the central cornea than the cone apex.
The uneven energy delivery with older CXL machines can be understood looking at demarcation line depth 10 to 15 days after CXL: The line is deeper in the center and gradually becomes superficial toward the periphery (Figure 2). This is not useful in terms of coma reduction because, in most cases of keratoconus, the apex is 2 mm away from the corneal vertex.
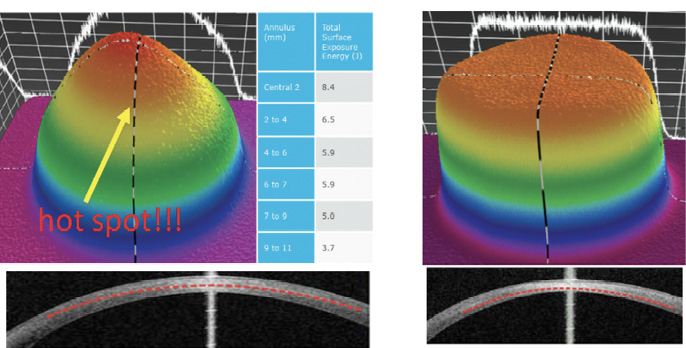
Figure 2. Uneven energy distribution and demarcation line during CXL performed with an old CXL machine (left). Homogeneous energy pattern and demarcation line in a cornea treated by a newer beam-optimized CXL machine (right).
Top-hat or umbrella-like beams produced by newer CXL machines significantly improve energy management, delivering a more even amount of radiation from center to periphery and, thus, improving the crosslinking effect in the apex zone (Figure 2).11 More than this, in the STARE-X CXL protocol, the beam is centered on the posterior elevation apex, eventually covering the limbus with a sponge if the beam diameter exceeds the corneal limbus. Demarcation line depth after this protocol is more even from center to periphery (Figure 2).11 A customized and cone-centered irradiation profile will lead to more effective crosslinking, more flattening in the apex zone, and a better refractive effect.
Fundamental 4: Consider the STARE-X protocol for ectatic corneas with a high degree of comatic aberration
The combination of accelerated CXL and corneal wavefront-guided transepithelial PRK should be considered in patients with a high degree of comatic aberration (root mean square, > 1 at 5 mm pupil diameter). We perform the STARE-X protocol using the Amaris excimer laser (Schwind eye-tech-solutions); the ablation is customized using corneal wavefront data acquired by the Sirius corneal tomograph (CSO), which combines a Scheimpflug camera, a Placido-disc topographer, and pupillometry. The KXL System (Avedro) is used to deliver the CXL treatment.
STARE-X protocol parameters for all treatments are as follows: maximum stromal ablation depth less than 50 μm; maximum optimization for saving ablation depth, as calculated by excimer laser software; maximum correction of coma aberration, eventually reducing spherical and cylinder refraction to not exceed the 50-μm ablation depth limit; epithelium removal of 55 μm in the center and 65 μm in the periphery of the selected ablation zone (we suggest to not extend over 6.5 mm to reduce ablation volume); and ablation offset up to 1 mm from corneal vertex in the direction of the cone apex as measured manually on topography. These settings allow the surgeon to center the ablation more on the cone with respect to the corneal vertex, reducing stromal ablation over the cone apex and further reducing coma aberrations (Figure 3).
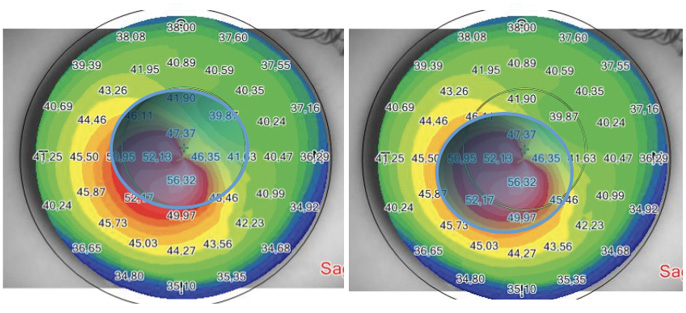
Figure 3. Corneal vertex–centered ablation (left) does not fully cover the apex zone and ablates tissue outside the corneal ectasia. Cone apex–centered ablation (right) fully covers the apex zone, saving nonectatic corneal tissue outside the cone.
Fundamental 5: Consider laser epithelium removal alone in thinner corneas with a high degree of aberration and a corneal apex greater than 53.00 D
In patients with keratoconus, the epithelium is normally thinner on the cone apex, acting as a masking agent for smoothing anterior elevation irregularities. Previous studies show an average thinning of 10 to 13 μm at 1.2 mm from the corneal vertex between normal corneas and those with keratoconus.12,13 This means that a 55- to 65-μm epithelial ablation in the apex zone will remove about 15 to 20 μm of stromal tissue. This is crucial information to consider, especially if removal of the epithelium or further topography-guided stromal regularization is planned, to not exceed the ablation depth target. This is the reason why epithelium removal alone will flatten the cone apex and reduce comatic aberrations (Figures 4 and 5).
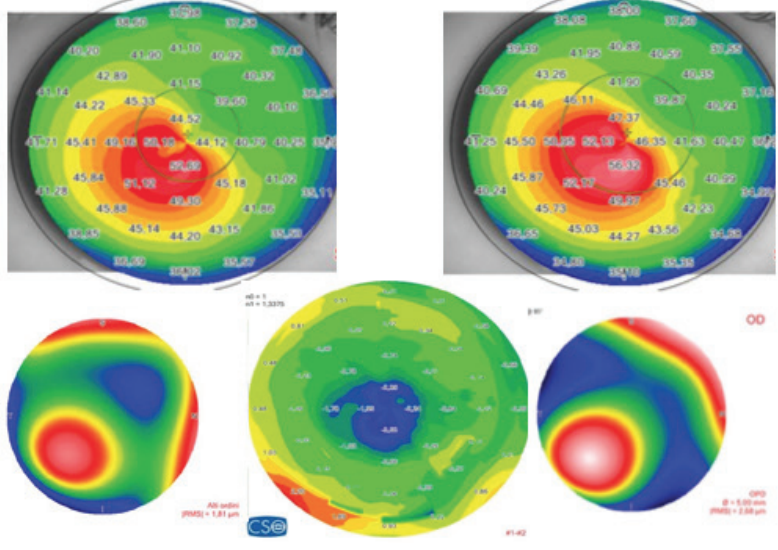
Figure 4. (Left) Preoperative sagittal map and aberration map (top and bottom right), postoperative (top and bottom left), and differential curvature map (bottom center) of a patient with keratoconus, thin corneas, and cone apex of more than 55.00 D treated with decentered laser epithelium removal plus accelerated CXL.
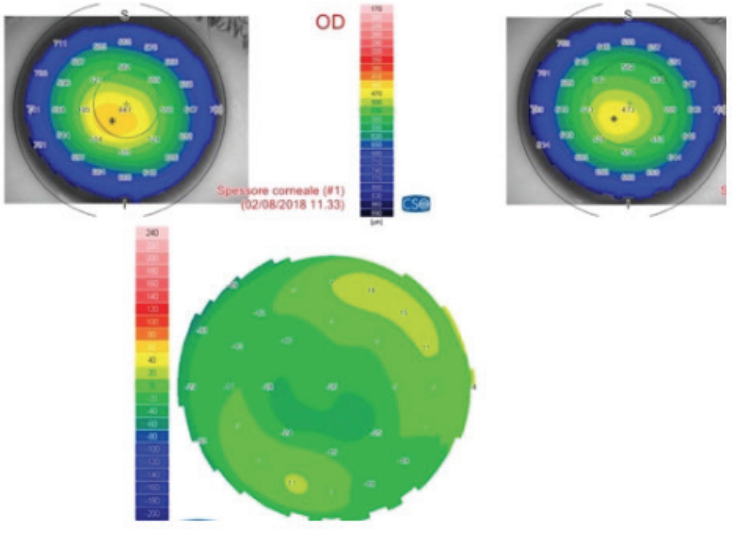
Figure 5. (Right) Pachymetric map of the patient in Figure 4. The differential map (bottom) shows corneal thinning of 24 µm in the apex zone 6 months after laser epithelium removal plus accelerated CXL.
Conclusion
CXL alone represents an optimal solution for early ectasia in which the goal is to halt the progression of the condition in patients with good BCVA and few higher-order aberrations, preventing the need for an eventual corneal transplant.
The next challenge lies in how to manage keratoconus in patients with significant ectasia. The refraction of these patients is always difficult because spectacle correction is unable to improve high irregular astigmatism, especially in peripheral cones, and a comfortable contact lens fitting is sometimes hard or impossible to achieve and can cause corneal scarring or infection.
The way to improve visual acuity in these patients is through corneal reshaping. In other words, the goal is to regularize the corneal geometry in the central 4-mm zone. This should reduce the vertical asymmetry and higher-order aberrations (especially coma) typically induced by ectasia.
The treatment of corneas with keratoconus using an excimer laser was historically considered inappropriate because it causes further corneal thinning and possible weakening of the corneal microstructure, leading to worsening of iatrogenic ectasia. Recent advances in technology have led to the development of corneal wavefront-guided treatment which has improved the quality of laser treatment and introduced the concept of customized treatments.
The benefit of combined CXL plus refractive surgery is seen in the early postoperative period, regularizing the ectatic cornea and treating the new, reshaped cornea with CXL to further flatten the cornea in the medium to long term.
1. Mazzotta C, Traversi C, Paradiso AL, et al. Pulsed light accelerated crosslinking versus continuous light accelerated crosslinking: One-year results. J Ophthalmol. 2014;2014:604731.
2. Mazzotta C, Baiocchi S, Bagaglia SA, et al. Accelerated 15 mW pulsed-light crosslinking to treat progressive keratoconus: Two-year clinical results. J Cataract Refract Surg. 2017;43(8):1081-1088.
3. Mazzotta C, Traversi C, Baiocchi S, et al. Corneal collagen cross-linking with riboflavin and ultraviolet A light for pediatric keratoconus: Ten-year results. Cornea. 2018;37(5):560-566.
4. Mazzotta C, Traversi C, Mellace P, et al. Keratoconus progression in patients with allergy and elevated surface matrix metalloproteinase 9 point-of-care test [published online ahead of print October 4, 2017]. Eye Contact Lens. 2017.
5. Wu H, Luo S, Dong N, et al. The clinical study of corneal cross-linking with hypo-osmolar riboflavin solution in thin keratoconic corneas. Zhonghua Yan Ke Za Zhi. 2014;50:681-686.
6. Mazzotta C, Caragiuli S. Intraoperative corneal thickness measurement by optical coherence tomography in keratoconic patients undergoing corneal collagen cross-linking. Am J Ophthalmol. 2014;157(6):1156-1162.
7. Rechichi M, Mazzotta C, Daya S, et al. Intraoperative OCT pachymetry in patients undergoing dextran-free riboflavin UVA accelerated corneal collagen crosslinking. Curr Eye Res. 2016;16:1-6.
8. Mazzotta C, Raiskup F, Baiocchi S, et al. Management of early progressive corneal ectasia. Heidelberg: Springer International; 2017.
9. Greenstein SA, Fry KL, Hersh PS. Effect of topographic cone location on outcomes of corneal collagen cross-linking for keratoconus and corneal ectasia. J Refract Surg. 2012;28(6):397–405.
10. Seiler TG, Fischinger I, Koller T, et al. Customized corneal cross-linking: one-year results. Am J Ophthalmol. 2016;166:14-21.
11. Crosslinking: past, present and future [eBook]. Avedro. 2013.
12. Haque S, Simpson T, Jones L. Corneal and epithelial thickness in keratoconus: a comparison of ultrasonic pachymetry, Orbscan II, and optical coherence tomography. J Refract Surg. 2006; 22:486-493.
13. Reinstein DZ, Gobbe M, Archer TJ, et al. Epithelial, stromal, and total corneal thickness in keratoconus: three-dimensional display with Artemis very-high frequency digital ultrasound. J Refract Surg. 2010; 26:259-271.