
Multiple needles, tips, instruments, and devices enter and leave the anterior chamber during cataract surgery. One major step in the procedure is removal of the hard nucleus, but equally important is the final step—introduction of the IOL into the capsular bag.
Each step of cataract surgery has evolved over the decades. A wire vectis for nucleus removal was replaced by phacoemulsification, and femtosecond lasers appear to be replacing the knife for incision creation.1-3 Similarly, the place of lens holding and folding forceps is increasingly being taken by myriad IOL insertion systems.
The landscape of cataract surgery was irrevocably changed when Sir Harold Ridley introduced the first IOL in 1949.4-6 Likewise, the introduction of phacoemulsification by Charles D. Kelman, MD, led to the era of small-incision cataract surgery.2,7 Another breakthrough in evolution occurred in the 1980s, when Thomas R. Mazzocco, MD, developed the first foldable IOL.8,9 This innovation allowed lens insertion without enlargement of the 3-mm phaco wound.10
IMPORTANCE OF INCISION SIZE
Like any other operation, cataract surgery begins and ends with the making and closing of an incision. The importance of a small incision size was realized early in the history of cataract/IOL surgery, and safe and effective delivery and implantation of a foldable IOL through a small incision has been a continual area of interest for phaco surgeons.
Manual holding, folding, and insertion techniques have, in recent years, been largely replaced by the use of novel IOL injection systems. The concept of dispensing an IOL as a prepackaged, ready-to-insert, preloaded IOL has been adopted by many manufacturers. The focus of these innovations is on development of simple, safe, and effective devices for IOL implantation through a relatively small incision.11,12
CHARACTERISTICS OF PRELOADED SYSTEMS
Preloaded IOL systems must be surgeon-friendly and should offer safety, ease of insertion, and time-saving in the surgical procedure.13 The characteristics of an ideal preloaded IOL injection system include the following:
- One-step, ready-to-insert implant package;
- Optimized design for a small incision size;
- Smooth passage of the IOL through the device with minimum friction;
- No risk of damage to IOL optic or haptics;
- Smooth folding and unfolding of the IOL; and
- Convenient and cost-effective use.
The benefits of a good preloaded IOL injection system are several. First, the delivery system enables consistent, predictable, and controlled insertion with minimal incision size. Second, it eliminates the need for the postoperative cleaning and sterilization associated with reusable systems. Third, it saves time in the operating room, and, fourth, it eliminates handling and misloading of the IOL that can occur with a manual tucking mechanism.

Figure 1. PhysIOL 123 System (courtesy of PhysIOL).

Figure 2. Quatrix IOL and its insertion system (courtesy of Croma-Pharma).

Figure 3. CT Asphina 603P IOL and its insertion system (courtesy of Carl Zeiss Meditec).
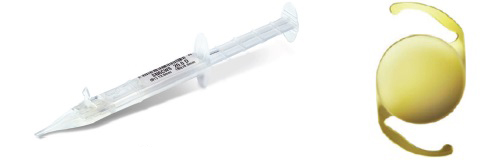
Figure 4. AcrySert C and AcrySof IQ IOL (courtesy of Alcon).
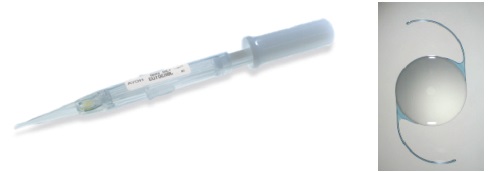
Figure 5. Isert 3P model and its insertion system (courtesy of Hoya).
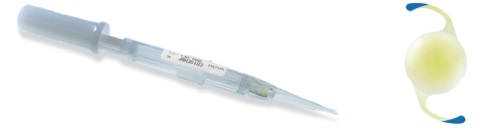
Figure 6. Isert 251 one-piece IOL and its injection system (courtesy of Hoya).
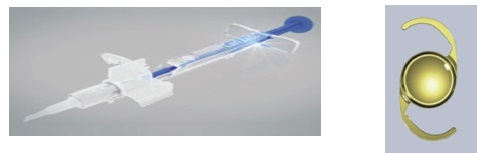
Figure 7. CT Lucia one-piece IOL and its injection system (courtesy of Carl Zeiss Meditec).
CLINICAL EXPERIENCE
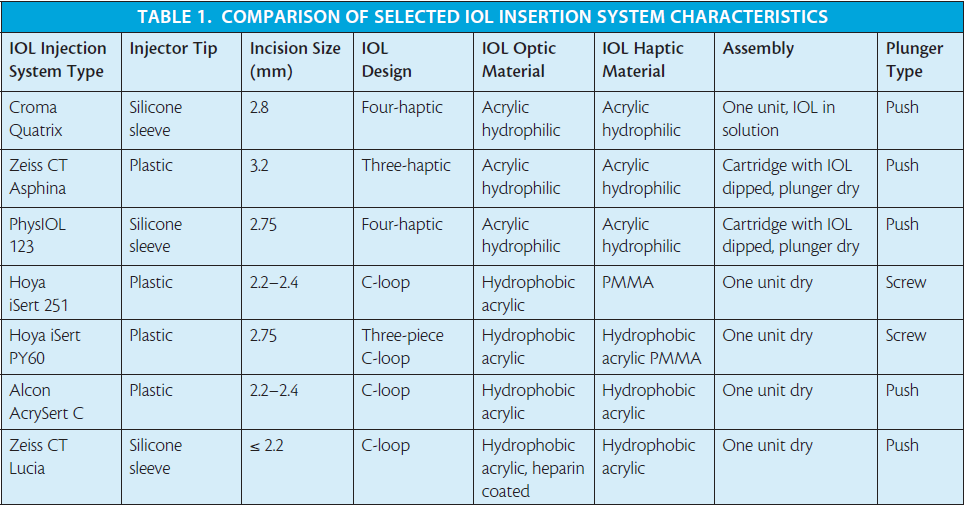
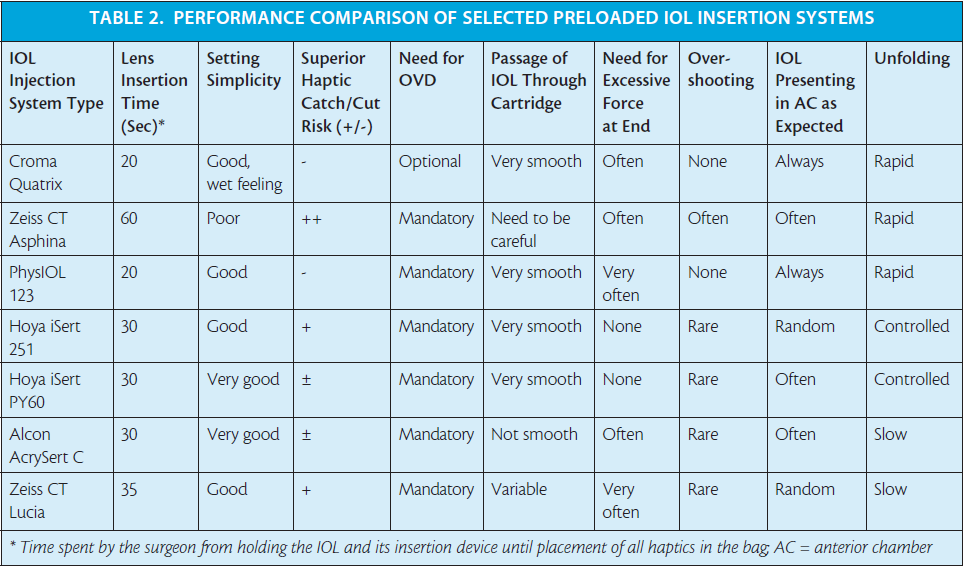
I have clinical experience with some of the preloaded insertion systems for both hydrophilic and hydrophobic IOL models (Tables 1 and 2 and Figures 1 through 7),4,14 and my clinical impressions are outlined in this article.
Hydrophilic preloaded IOL systems. The IOL insertion system for use with the Micro A and Micro AY IOLs (PhysIOL) is called the PhysIOL 123 system (Figure 1). The systems for the Quatrix IOL (Croma-Pharma; Figure 2), and the CT Asphina 603P (formerly XL Stabi ZO; Carl Zeiss Meditec; Figure 3) IOLs do not have designated brand names. In the PhysIOL 123 and Zeiss systems, the IOL is packaged separately from its cartridge and plunger. The Chroma IOL and inserter system are dispensed as one unit. With the PhysIOL system, the IOL is assembled into the delivery unit with a no-touch technique, and, aside from this step, it is the most effective IOL delivery system in my experience.
The Chroma Quatrix IOL comes out of the inserter completely hydrated, as it is packaged in balanced saline solution. Both the Chroma and PhysIOL devices include a silicone sleeve to facilitate pushing the IOL through a narrow cartridge canal.
The Zeiss IOL inserter performed poorly in our hands. The disadvantages with this system included cumbersome assembly and unlocking and the use of a plastic plunger to push the IOL through the cartridge. This design factor increased the risk of haptic catch. I have compared the injection techniques for these three preloaded hydrophilic IOL systems in a video available on http://eyetube. net/?v=ducha.
Hydrophobic preloaded IOL systems. More recently, I had the opportunity to use three preloaded systems for hydrophobic IOLs: the AcrySert C inserter for the AcrySof IQ IOL (Alcon; Figure 4); the iSert for Hoya one- and three-piece IOLs (Hoya; Figures 5 and 6); and the inserter for the CT Lucia one-piece IOL (Carl Zeiss Meditec; Figure 7).
Watch it Now
Easy Insertion With Preloaded IOLs
Dr. ul Mazhry compares injection techniques for three preloaded IOL systems: PhysIOL123, Quantrix (Croma), and CT Asphina 603P (Carl Zeiss Meditec).
The iSert received my best rating, owing to its easy handling and fail-safe delivery through a 2.75-mm incision. The AcrySert C was also good but lagged far behind the already established Monarch 2 and 3 systems (Alcon) for smooth intraocular delivery of the AcrySof IOL. (Editor’s Note: Since the writing of this article, Alcon has introduced the UltraSert Preloaded Delivery System for the AcrySof IQ Aspheric IOL.)
The Zeiss CT Lucia system is supposed to deliver the IOL through an incision of less than 2.2 mm. However, this narrows the cartridge markedly at its preinjection position. One needs to apply excessive force to inject the lens, and my preliminary results showed increased risk of IOL haptic catch or damage.
At a Glance
• The concept of dispensing an IOL as a prepackaged, ready-to-insert, preloaded IOL has been adopted by many manufacturers.
• There are four key benefits of a good preloaded IOL injection system: (1) enabling consistent, predictable, and controlled insertion with minimal incision; (2) eliminating the need for the postoperative cleaning and sterilization associated with reusable systems; (3) saving time in the operating room; and (4) eliminating handling and misloading of the IOL that can occur with a manual tucking mechanism.
• IOL manufacturers are keenly working on the development of better preloaded IOL delivery systems to make IOL size, design, and thickness more compatible with the disposable plunger and cartridge assembly.
The four-haptic design of hydrophilic IOLs such as the Croma Quatrix appeared to be more compatible with preloaded insertion systems; however, these lenses are associated with a higher incidence of posterior capsular opacification (PCO), pigment deposition, and lens discoloration. The preloaded IOL insertion systems for hydrophobic C-loop one- and three-piece designs need more work to ensure fail-safe delivery; however, these lenses definitely perform better in terms of long-term lens clarity and less PCO.15-17
FUTURE TRENDS
IOL manufacturers are keenly working on the development of better preloaded IOL delivery systems. The focus is on making IOL size, design, and thickness more compatible with the disposable plunger and cartridge assembly.
I believe that, in the near future, all IOL implantations will be performed through preloaded IOL delivery systems. The healthy competition among manufacturers to achieve the best system will not only provide us with flawless IOL delivery but will also make these products more cost-effective. n
1. Moore DB, Harris A, Siesky B. Republished review: The world through a lens: the vision of Sir Harold Ridley. Postgrad Med J. 2011;87(1026):307-310.
2. Packer M. Phaco has turned 40, gracefully. Expert Rev Ophthalmol. 2008;3(2):113-116.
3. He L, Sheehy K, Culbertson W. Femtosecond laser-assisted cataract surgery. Curr Opin Ophthalmol. 2011;22(1):43-52.
4. Mazhry Z. Comparison between two aspheric preloaded IOL insertion systems. Paper presented at ESCRS annual meeting; September 17-231, 2011; Vienna, Austria.
5. Trivedi RH, Apple DJ, Pandey SK, et al. Sir Nicholas Harold Ridley. He changed the world, so that we might better see it. Indian J Ophthalmol. 51(3):211-216.
6. Barraquer J. Cataract surgery and IOL implantation. More than 40 years of personal experience. My present criteria and considerations. Doc Ophthalmol. 1992;81(3):267-280.
7. Pandey SK, Milverton EJ, Maloof AJ. A tribute to Charles David Kelman MD: Ophthalmologist, inventor and pioneer of phacoemulsification surgery. Clin Exp Ophthalmol. 2004;32(5):529-533.
8. Boyle E. Foldable IOLs ushered in new cataract and refractive paradigm. Ocular Surgery News. June 1, 2007.
9. Devices for implantation of deformable intraocular lens structures. December 1987. http://www.google.com/patents/US4715373. Accessed July 11, 2015.
10. Packard R. Implantology: the evolution from large rigid to small foldable IOLs. CRST Europe. January 2014.
11. Masket S, Wang L, Belani S. Induced astigmatism with 2.2- and 3.0-mm coaxial phacoemulsification incisions. J Refract Surg. 25(1):21-24.
12. Oshika T, Nagahara K, Yaguchi S, et al. Three year prospective, randomized evaluation of intraocular lens implantation through 3.2 and 5.5 mm incisions. J Cataract Refract Surg. 1998;24(4):509-514.
13. Albe E, Trattler WB, Awdeh RM. An eyeball space odyssey. CRST Europe. April 2011.
14. Mazhry Z. Experience with preloaded IOLs. Paper presented at ESCRS annual meeting; September 17-231, 2011; Vienna, Austria.
15. Buehl W, Findl O, Menapace R, et al. Long-term effect of optic edge design in an acrylic intraocular lens on posterior capsule opacification. J Cataract Refract Surg. 2005;31(5):954-961.
16. Kugelberg M, Wejde G, Jayaram H, Zetterström C. Two-year follow-up of posterior capsule opacification after implantation of a hydrophilic or hydrophobic acrylic intraocular lens. Acta Ophthalmol. 2008;86(5):533-536.
17. Kugelberg M, Wejde G, Jayaram H, Zetterström C. Posterior capsule opacification after implantation of a hydrophilic or a hydrophobic acrylic intraocular lens. One-year follow-up. J Cataract Refract Surg. 2006;32(10):1627-1631.
Zia ul Mazhry, FRCS(Ed), FRCS (Glas), FCPS (Pakistan), CIC Ophth (Lond)
• Associate Professor, Central Park Medical College, Lahore, Pakistan
• Consultant Eye Surgeon and Head of Department, Wapda Teaching Hospital Complex, Lahore, Pakistan
• mazhry@hotmail.com
• Financial disclosure: None
