



Customized laser vision ablation using ocular wavefront (OWF) aberration data1 benefits from the measurement of ocular higher-order aberrations (HOAs) with devices such as a Hartmann-Shack aberrometer.2 Corneal WF (CWF) maps are derived from corneal topography to better explain and quantify corneal irregularities.3,4 The use of CWF maps to treat eyes with corneal irregularities shares the advantages of topography-guided (TG) ablations and adds the choice of aberrations to create a slimmer ablation profile and spare tissue.5
WF-GUIDED TREATMENTS
The optical quality of an eye is determined by its WF aberrations. Whether corneal or ocular, WF aberrations define optical errors. WF-guided (WFG) treatment is based on the measured CWF or OWF data and is thus highly individualized and patient-oriented. WF correction can be achieved by applying the reverse WF. Because a laser system can remove tissue but not add it, the WF correction must also consider shifting the ablation profile to prevent negative ablation values. The correction is performed by modifying the anterior surface of the cornea with photoablation.
Patients with a history of radial keratotomy have significant corneal irregularities. TG- and WFG-PRK can significantly improve their visual acuity (Figure 1).
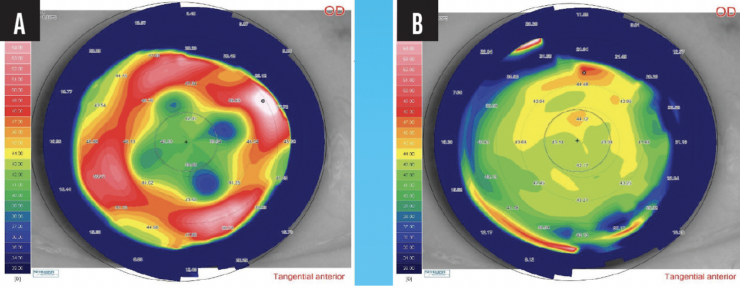
Figure 1. Preoperative (A) and 12-month postoperative (B) corneal topography of a patient treated with TG-PRK who had undergone radial keratotomy 35 years earlier.
OWF-GUIDED TREATMENTS
The goal of OWF-guided treatment is to eliminate or reduce the total aberrations of an eye. An advantage of OWG treatment is that it is based on an objective refraction of the complete optical system. A high-resolution measurement, typically more than 800 points for a 7-mm pupil for Hartmann-Shack systems or more than 25,000 points for pyramidal sensors,6,7 is used to design ablation profiles to compensate for the OWF aberrations. Critics of this approach have argued that aberrations, especially those of the crystalline lens, change significantly with accommodation and with age.
PRK can achieve significant improvements in the corneal topography and visual acuity of patients with myopia (Figure 2).
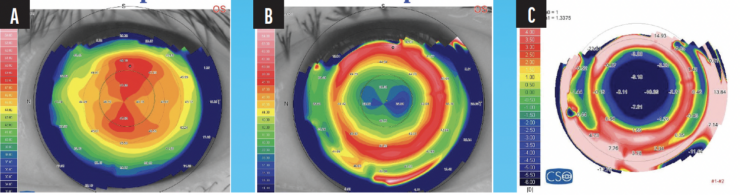
Figure 2. Preoperative (A) and 6-month postoperative (B) corneal topography of a patient with extreme myopia treated with PRK. The subtraction map shows the changes in corneal topography following the procedure (C).
PERFORMING CWF-GUIDED TREATMENT: A STEP-BY-STEP APPROACH
With CWF-guided treatment, the approach is tailored to the eye, and stromal tissue is spared when possible to minimize overcorrections and refractive surprises. Following are broad guidelines for the clinician to follow when planning WFG treatment in general and a corneal WFG ablation specifically:
Step No. 1: Perform the manifest refraction (MRx).
Step No. 2: Obtain topographic or tomographic measurements.
Step No. 3: Transform topographic/tomographic data into CWF data.
Step No. 4: Measure the OWF.
Step No. 5: Compare the CWF and OWF data.
Step No. 6: Proceed with the CWG treatment plan if the relevant level of HOAs is present in the CWF and not in the internal WF. Otherwise, opt for OWG (relevant level of HOAs present in the OWF with relevant HOAs for internal WF) or an aberration-neutral profile (as a fallback approach).
Step No. 7: Modify (if necessary) and enter the refraction into the WFG treatment plan. The cylinder may be modified by considering the MRx, the amount of astigmatism measured at 4 mm with an aberrometer, the WF or WF astigmatism measured at 4 and 6 mm, and the topographic astigmatism. The sphere can be adjusted by evaluating the manifest spherical refraction and the aberrometer sphere at the 4- and 6-mm optical zones (OZs).
Step No. 8: Generate ablation plans for both conventional and customized treatments for the same eye and compare their depth and volume. If the difference is less than or equal to 0.75 D of equivalent tissue depth or volume for any given OZ, or if a narrower ablation is not desired, proceed to Step No. 11. A difference of more than 0.75 D would correspond to a difference in ablation depth greater than 10 µm for a 6-mm OZ (155 nL in volume for myopic ablations and 190 nL for hyperopic ablations), greater than 12 µm for a 6.5-mm OZ (215 nL in volume for myopic ablations and 260 nL for hyperopic ablations), and greater than 14 µm for a 7-mm OZ (300 nL for myopic and 350 nL for hyperopic ablations). Larger OZs are preferred whenever possible.
Step No. 9: Refine the refraction (sphere, cylinder, axis) to reduce the volume or depth of tissue ablated. You can adjust the refractive constraints to adjust the amount of tissue saved vis-à-vis the residual refraction.
Step No. 10: Allow the software to deselect specific HOAs from the Zernike pyramids to achieve a shallower ablation or remove less stromal tissue. Alternatively, deselect specific aberrations based on the clinical case.
Step No. 11: Review the final treatment plan and match it with the initial topography/tomography or OWF map depending on whether the chosen plan is CWG or OWG, respectively.
Step No. 12: If performing PRK, consider a transepithelial approach, which offers several advantages. The most important of these is the ability to treat what is measured by aberrometry or tomography because the anterior epithelial surface topography is translated down to the stroma by excimer laser phototherapeutic keratectomy. An OCT epithelial pachymetry map is a valuable tool to adjust the phototherapeutic keratectomy ablation depth, centrally and peripherally.
Step No. 13: Load treatment plan into the excimer laser platform.
FINAL THOUGHTS
The proper definition of an optimal ablation profile for corneal refractive surgery is a subject of debate. Considerations such as treatment duration, the amount of tissue removed, postoperative remodeling, and overall surgical outcomes have made it difficult to establish a universal optimal profile. These factors are interrelated and may lead to overcorrections, refractive surprises, ectasia, and treatment regression. WF-customized treatments are successful only if the preexisting aberrations exceed the repeatability threshold and the biologic noise. Additionally, the operative and postoperative factors mentioned can limit the accuracy of a given treatment.
In cases of post-LASIK ectasia, TG or WFG transepithelial PRK combined with CXL can effectively flatten the cornea and improve the patient’s visual acuity (Figure 3).
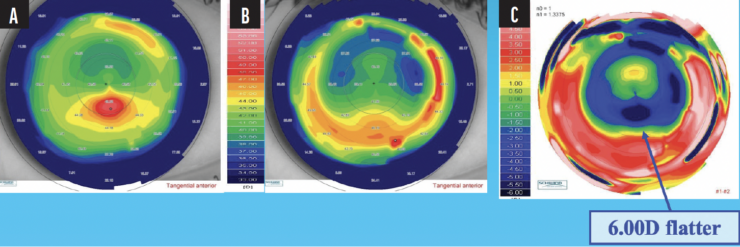
Figure 3. Preoperative (A) and 12-month postoperative (B) corneal topography of a 30-year-old patient with post-LASIK ectasia treated with TG-PRK and CXL. The subtraction map shows 6.00 D of flattening (C).
As a broad guideline, it makes sense to treat clinically relevant aberrations and opt for selective CWG ablation if most of the aberrations are on the cornea, especially when the scotopic pupil is smaller than the target aberrated corneal surface. CWG ablation offers the advantage of a shallower ablation compared to traditional TG treatment. In instances when the WF and ablation profiles of the CWG and OWG approaches differ, and given an adequate scotopic pupil size, OWG treatment may be preferable because it incorporates the MRx into the measured WF of the whole eye, improving postoperative refractive predictability.
1. Mrochen M, Kaemmerer M, Seiler T. Clinical results of wavefront-guided laser in situ keratomileusis 3 months after surgery. J Cataract Refract Surg. 2001;27(2):201-207.
2. Liang J, Grimm B, Goelz S, Bille JF. Objective measurement of wave aberrations of the human eye with the use of a Hartmann-Shack wave-front sensor. J Opt Soc Am A Opt Image Sci Vis. 1994;11(7):1949-1957.
3. Salmon TO. Corneal contribution to the wavefront aberration of the eye. Dissertation. Bloomington Indiana University; 1999.
4. Mrochen M, Jankov M, Bueeler M, Seiler T. Correlation between corneal and total wavefront aberrations in myopic eyes. J Refract Surg. 2003;19(2):104-112.
5. Arba-Mosquera S, Arbelaez MC, Merayo-Llovés J. Six-month clinical outcomes of customized treatments minimized for depth and time in laser corneal refractive surgery. Cornea. 2011;30(8):876-888.
6. Akondi V, Castillo S, Vohnsen B. Digital pyramid wavefront sensor with tunable modulation. Opt Express. 2013;21(15):18261-18272.
7. Plantet C, Meimon S, Conan JM, Fusco T. Revisiting the comparison between the Shack-Hartmann and the pyramid wavefront sensors via the Fisher information matrix. Opt Express. 2015;23(22):28619-28633.


