CASE PRESENTATION
A 38-year-old woman has an online consultation for refractive correction. The patient’s manifest refraction is -7.50 -3.00 x 180º = 1.25 OD and -7.25 -3.00 x 171º = 1.25 OS. Based on this information, implantation of a toric phakic IOL is recommended.
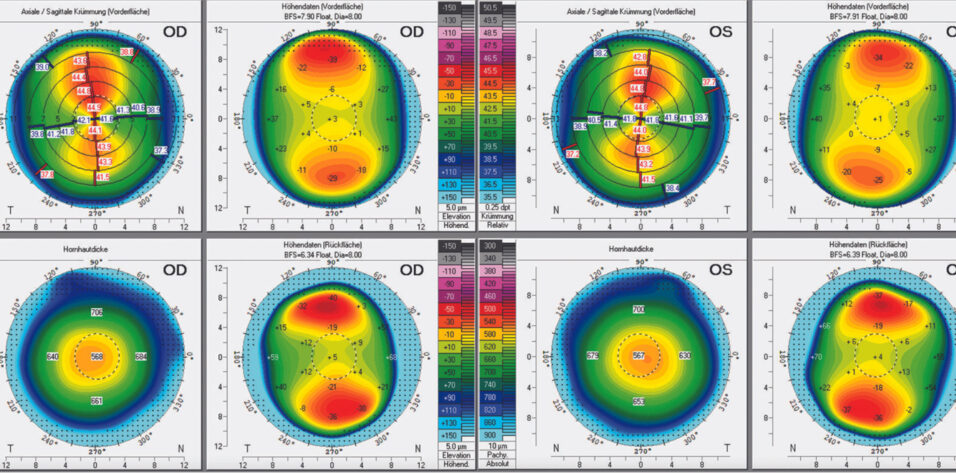
During a subsequent detailed examination at the clinic, the Pentacam (Oculus Optikgeräte) finds an unexpectedly shallow anterior chamber in each eye (2.52 mm OD and 2.49 mm OS) and narrow anterior chamber angles (29º OD and 31º OS; Figures 1 and 2). The Belin/Ambrósio Enhanced Ectasia Display final D scores are 0.88 OD and 0.79 OS. The corneal biomechanical index scores with the Corvis ST (Oculus Optikgeräte) are 0.01 OD and 0.00 OS, resulting in total biomechanical index scores of 0.21 OD and 0.01 OS.

Figure 1. Pentacam refractive maps show normal corneas.

Figure 2. Tomography of the anterior segment reveals a narrow angle and shallow anterior chamber in each eye.
What is your preferred approach to patients with emerging presbyopia and a similar prescription? What regulatory limits for phakic IOL implantation or laser vision correction apply in your country? How would you handle this case?
—Case prepared by Suphi Taneri, MD, FEBOS-CR

JORGE L. ALIÓ, MD, PHD, FEBOphth
A phakic IOL is usually my first choice for cases like this one, but I would not recommend it here because of the inadequate dimensions of the anterior chamber. The high risk of complications related to the induction of vitreous detachment followed by retinal detachment also makes refractive lensectomy unethical in patients of this age who have myopia.1-3
In this case, the topography of both eyes is normal with a central corneal thickness of 568 µm OD and 567 µm OS. SMILE with an optical zone of 6.5 mm would leave a residual stromal bed of 279 µm OD and 261 µm OS. The procedure is therefore a reasonable alternative for both eyes. The only limitation is the relative risk of undercorrected astigmatism after SMILE because the preoperative cylinder power is greater than 2.00 D and the chance that retreatment will be required is high.4
LASIK is a reasonable option as well. A 100-µm flap would leave a percentage of tissue altered (flap thickness + ablation depth/central corneal thickness) of 42% in each eye. A 6-mm optical zone with transition zones of 7.96 and 7.93 mm would be used in the right and left eyes, respectively, with the Schwind Amaris 750 (Schwind eye-tech-solutions).
SMILE would be my first choice for the patient. Although definitive supporting evidence is lacking, based on theoretical tenets,5 I believe that SMILE should have less of a biomechanical impact than LASIK. My only concern is the 3.00 D of astigmatism, which will probably be undercorrected. The patient would have to agree to accept this possibility because a retreatment is not an option. LASIK would be my recommendation if the patient wants greater refractive precision in the outcome. The safety and stability of LASIK for the treatment of high myopia is well established.6
Doctors should be willing to say no to some patients. A reasonable option in the current case is to decline to perform refractive surgery. A surgeon whose excimer laser system ablates more tissue per diopter than I have described (eg, top-hat laser beam configurations) or has fewer ablative capabilities should abandon the idea of corneal refractive surgery because an excessive amount of tissue would be consumed.

ROGER ZALDIVAR, MD, MBA
The options available at my practice are all borderline because of the patient's characteristics. Despite a spherical equivalent of 9.00 D OU, LASIK is worth considering. In situations like this one, I analyze pupil size under different lighting conditions, specifically mesopic and scotopic. I try to avoid the combination of a high-correction ablation and a large pupil, which can result in a larger expression of spherical aberration with possible implications for quality of vision.
I am uncomfortable with performing surface ablation on patients like this one due to the increased risk of postoperative haze, so I would not offer it here.
An off-label option would be to implant an EVO Toric ICL (STAAR Surgical) bilaterally. Even though the anterior chamber depth is far from the accepted 2.8 mm, both measured angles are greater than 30º. I have implanted the EVO in eyes with shallow chambers (Figure 3), achieved excellent outcomes, and tracked the patients over time with no complications.

Figure 3. An EVO ICL in an eye with a shallow chamber.
(Figures 3 and 4 courtesy of Roger Zaldivar, MD, MBA)
In extreme cases such as this one, attending to every detail is crucial to obtain a safe vault. Because the spherical equivalent is less than 10.00 D, the lens would have only one posterior curvature (Figure 4). This would make predicting the peripheral vault easier. Higher-powered ICLs have a thicker periphery and a more compromised safety zone (residual angle and vault) in a shallow chamber.

Figure 4. A low-powered ICL with one homogenous posterior radius of curvature.
I perform ultrasound biomicroscopy (UBM) on every eye in which I implant an EVO ICL. UBM-based sizing methodologies have been shown to be accurate for predicting vault. My colleagues and I use a unique sizing method that is based on different UBM measurements and accounts for multiple anatomic components of the eye and the lens to be implanted. Of our most recent 150 cases at the time of this writing, 61% were within 100 µm, 86% within 200 µm, and 99% within 300 µm of vault predictability.
If the patient were 10 years older, I would probably postpone surgery until she experiences a vitreous detachment and then perform a refractive lens exchange with a toric extended depth of focus IOL.

WHAT I DID: SUPHI TANERI, MD, FEBOS-CR
This was a tricky case. As the panelists note, several treatment options are available, but none is perfect. In Germany, where I practice, myopia with more than -10.00 D of astigmatism is considered outside the treatment range of laser vision correction. Normally, I implant a (toric) phakic IOL in eyes like these. An examination, however, revealed a shallow anterior chamber and relatively small chamber angle; a 2.8-mm internal chamber depth is the minimum requirement. The patient had clear crystalline lenses and was not yet presbyopic, so refractive lens exchange was contraindicated. Fortunately, the corneas seemed ideal for laser vision correction.
After an extensive preoperative discussion of the options and their risks and benefits, the patient elected to proceed with bilateral lenticule extraction (SMILE). A 6.5-mm lenticule diameter was used in each eye. Four months after surgery, her UCVA was 1.6 OU. Her BCVA was 1.6 OU with a manifest refraction of 0.50 D OD and +0.25 - 0.50 x 143º OS. Two years after surgery, the corneas were stable. Her UCVA was 1.0 OD and 1.25 OS. Her BCVA was 0.50 -1.00 x 33º = 1.6 OD and +0.37 -0.75 x 150º = 1.25 OS.
1. Alió JL, Grzybowski A, El Aswad A, Romaniuk D. Refractive lens exchange. Surv Ophthalmol. 2014;59(6):579-598.
2. Alió JL. Lens surgery (cataract and refractive lens exchange) and retinal detachment risk in myopes: Still an issue? Br J Ophthalmol. 2011;95(3):301-303.
3. Figueroa MS, Govetto A. Retinal detachment. In: Alió JL, Azar D, eds. Management of Complications in Refractive Surgery. 2nd ed. Springer; 2018:311-314.
4. Alió Del Barrio JL, Vargas V, Al-Shymali O, Alió JL. Small incision lenticule extraction (SMILE) in the correction of myopic astigmatism: outcomes and limitations – an update. Eye Vis (Lond). 2017;4:26.
5. Reinstein DZ, Archer TJ, Randleman JB. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg. 2013;29(7):454-460.
6. Alió JL, Muftuoglu O, Ortiz D, et al. Ten-year follow-up of laser in situ keratomileusis for myopia of up to -10 diopters. Am J Ophthalmol. 2008;145(1):46-54.