DAVID F. CHANG, MD
There are several useful diagnostic technologies, but one that I find indispensable is the iTrace (Tracey Technologies). In addition to being a topographer, the iTrace simultaneously performs wavefront aberrometry. Most important, it measures and calculates the higher-order aberrations (HOAs) of the cornea separately from those of the internal optics (eg, the lens). Additionally, it performs pupillometry and quantifies the HOAs that the patient would see through that specific pupil diameter. For example, corneal irregularity measured over a standard 6-mm diameter zone may not be problematic with a 2-mm pupil. Knowing this information is important if a multifocal IOL is considered, and I have decided against using a diffractive multifocal lens in many cases because of the corneal HOAs measured through a particular patient’s actual pupil size in a dimly lit room.
Finally, the iTrace topographer not only provides a traditional 3-mm simulated keratometry (SimK) reading but also maps the refractive keratometry (K). By totaling data from 600 individual ray tracings through the central 3-mm zone, it gives a best-fit K reading that is quite often different from the traditional K reading from a single 3-mm ring. This is helpful with some astigmatic patterns, particularly post-LASIK patterns. The iTrace also automatically measures angle kappa and angle alpha and has a unique toric IOL planner. An entire bilateral exam takes only a few minutes, and the machine footprint is like that of any topographer.
David F. Chang, MD, is a Clinical Professor at the University of California, San Francisco, and in private practice in Los Altos, California. Dr. Chang is a member of the CRST Europe Global Advisory Board and states that he has no financial interest in any instrument or technique he describes. He may be reached at e-mail: dceye@earthlink.net.
ARTHUR B. CUMMINGS, MB ChB, FCS(SA), MMed(Ophth), FRCS(Edin)
I do the following diagnostic tests routinely: corneal topography, corneal tomography, and optical coherence tomography (OCT) of the macula and optic nerve. On the odd occasion, however—if the clinical picture warrants it—I add iTrace topography and aberrometry and Cassini corneal topography (i-Optics).
Corneal topography is essential to determine any irregular astigmatism and identify a large angle kappa, two clinical conditions that will prevent me from using multifocal IOLs. Corneal tomography is also valuable in terms of the above, but in addition it provides more anterior chamber dimensions in terms of angle, depth, and volume. Knowing these things can prompt me to select an IOL solution over LASIK or other corneal refractive surgical solutions if the dimensions are sufficiently reduced. Knowing the toricity of the posterior corneal surface also helps with the choice of a toric IOL.
I typically try to obtain an OCT of the macula and optic nerve for all patients undergoing cataract or clear lens extraction surgery, not just those who receive premium IOLs. I like to see the state of the nerve fiber layer and the macular retinal pigment epithelium, and I am also keen on seeing the state of the posterior vitreous and whether there has been a total posterior vitreous detachment or there is some vitreomacular traction present. I have also diagnosed many epiretinal membranes on OCT that I simply never saw on fundus exam at the slit lamp using 78.00 or 90.00 D lenses. These factors all impact the timing of surgery and the choice of IOL.
[Courtesy of Arthur B. Cummings, MB ChB, FCS(SA), MMed(Ophth), FRCS(Edin)]
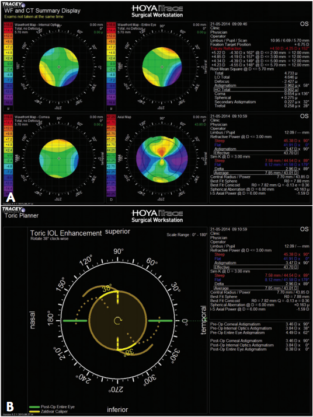
Figure 1. Postoperative iTrace maps (A) showing entire eye’s wavefront (top right) and corneal wavefront (bottom left). The effect of the toric IOL is shown top left. Ideally, the toric IOL and corneal astigmatism axes should coincide in order to fully address the refractive astigmatism. This IOL must be rotated 38º clockwise in order to gain the full effect of the toric IOL (B).
In eyes with significant astigmatism, the Cassini provides another reliable source for magnitude and axis of corneal astigmatism. This may alter the axis that I use for toric IOL calculations. When there is significant refractive astigmatism present, I like using the iTrace in order to determine the amount of corneal lenticular astigmatism. The iTrace is particularly helpful when the refractive result is not as good as expected after toric IOL implantation (Figure 1A). Using the iTrace here can help the surgeon determine how the IOL should be rotated in order to improve the refractive outcome (Figure 1B).
Today’s cataract surgery has come a long way from the “Wham, bam, thank you ma’am” days; now, it is akin to a proper refractive surgery work-up in our clinic.
Arthur B. Cummings, MB ChB, FCS(SA), MMed(Ophth), FRCS(Edin), is a Consultant Ophthalmologist at the Wellington Eye Clinic and UPMC Beacon Hospital in Dublin, Ireland, and an Associate Chief Medical Editor of CRST Europe. Dr. Cummings states that he has no financial interest in any instrument or technique he describes. He may be reached at e-mail: abc@wellingtoneyeclinic.com.
ALOIS K. DEXL, MD, MSc
Within the past decade, cataract surgery has rapidly changed from a simple procedure involving extraction and replacement of the clouded lens to a complex refractive procedure demanding the best possible postoperative results in order to achieve patient satisfaction. When it comes to refractive surprises after cataract surgery, an ounce of prevention is worth a pound of cure, and surprises can be anticipated in certain patients. From my point of view, the most essential test is to talk with each patient in order to avoid unrealistic expectations.
Apart from a complete ophthalmologic examination, accurate biometry with a modern partial coherence interferometry (PCI) device, such as the IOLMaster 500 (Carl Zeiss Meditec) or the Lenstar LS900 (Haag-Streit), is one key to success. To assure the regularity of potential corneal astigmatism, corneal topography or tomography is also beneficial. With conventional IOL power calculations, even when the appropriate formulas for extreme myopes and hyperopes are used, patients can end up hyperopic. We must adjust our calculations for extreme cases, targeting slight residual myopia.
We try to get patients as close as possible to their refractive goal, but we do not know exactly what we will achieve until after surgery. This is especially true for very long or short eyes. When a patient presents with bilateral cataract, I typically perform surgery in the nondominant eye first and explain that, in the event of a refractive surprise in the first eye, I can adjust the implant power in the dominant eye to achieve vision closer to target. It is rare to encounter a patient who does not tolerate 1.00 D of myopic anisometropia, but it happens. In such a case, I discuss with the patient how to proceed.
Alois K. Dexl, MD, MSc, is an Associate Professor in the Department of Ophthalmology, Paracelsus Medical University of Salzburg, Austria. Dr. Dexl states that he has no financial interest in any instrument or technique he describes. He may be reached at e-mail: a.dexl@salk.at.
ERIC D. DONNENFELD, MD
The goal of refractive cataract surgery is to provide safe and effective visual rehabilitation to the patient undergoing the operative procedure. The visual opportunity offered to the patient should be tempered by his or her specific needs as well as his or her eye’s preexisting pathology and anatomy.
Testing begins with optical or immersion biometry, either of which is markedly more accurate than a contact A-scan. I perform topography on all patients, even those without refractive cylinder, as the presence of forme fruste keratoconus and irregular astigmatism is often a relative or absolute contraindication to a multifocal IOL and changes my surgical approach with a toric IOL. Additionally, topography frequently demonstrates the presence of ocular surface disease and the need for tear film management. Ocular surface disease commonly reduces patient satisfaction following cataract surgery; identifying it preoperatively can allow the surgeon to develop an intelligent treatment plan.
For patients undergoing refractive cataract surgery, I routinely perform tear osmolarity and matrix metalloproteinase 9 testing and use the supravital stain lissamine green to look for conjunctival and corneal pathology. I perform a macular OCT on all patients requesting a multifocal IOL, as the loss of contrast sensitivity in these patients may be magnified by concomitant retinal disease.
Finally, I perform specialized testing in patients with underlying pathology to assess the severity of disease. In patients with glaucoma, I routinely obtain an optic nerve OCT to assess the nerve fiber layer, and in patients with complex cataract surgery, reoperations, or underlying cornea guttata I obtain a preoperative endothelial cell count.
With the intelligent use of preoperative testing, the surgeon can improve visual outcomes and patient satisfaction with refractive cataract surgery and more accurately counsel patients preoperatively about postoperative expectations.
Eric D. Donnenfeld, MD, is a Professor of Ophthalmology at New York University and a trustee of Dartmouth Medical School in Hanover, New Hampshire. Dr. Donnenfeld is a member of the CRST Europe Global Advisory Board. He states that he has no financial interest in any instrument or technique he describes. Dr. Donnenfeld may be reached at tel: +1 516 766 2519; e-mail: ericdonnenfeld@gmail.com.
OLIVER FINDL, MD, MBA, FEBO
Several measurements are required to achieve the desired refraction after cataract surgery. First and foremost, accurate measurements of the eye’s axial length are necessary. PCI is more accurate than ultrasound,1,2 and the latter should serve only as a back-up for the less than 5% of cases in which PCI is unsuccessful.3 The use of PCI instead of ultrasound can reduce the rate of postoperative refractive error from 17% to less than 3%.4
It is also essential to have a precise measurement of the corneal radii, especially for toric IOLs.5 In my hands, a combination of devices appears to be superior to a single measurement method. Keratometry is robust and useful, but it cannot give a detailed view of corneal irregularities. This information can be obtained by using Placido-disc topography measurements. One disadvantage of keratometry and topography, however, is that they account only for the anterior corneal surface, not the posterior surface, which contributes about 20% to the refractive power. To measure the entire cornea, tomographic devices are necessary. As we have shown recently, modern swept-source Fourier-domain OCT results in the most reliable measurements and will likely be a good choice in the future.
[Courtesy of Oliver Findl, MD, MBA, FEBO]
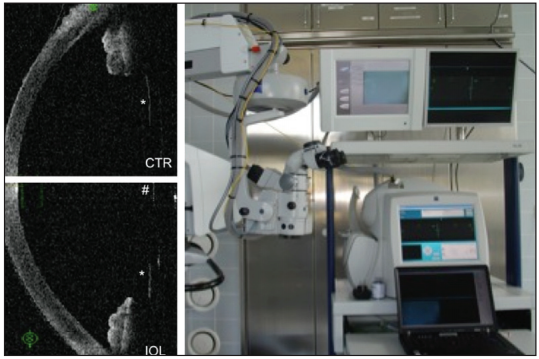
Figure 2. Continuous intraoperative OCT: Measurement of the capsule position in the aphakic eye (upper left) and after IOL implantation (lower left) with a prototype OCT attached to the operating microscope (right) can help to better predict postoperative IOL position.
Another aspect is the prediction of the postoperative IOL position, which appears to be the most crucial for postoperative refraction. Until now, this prediction has been done with IOL power calculation formulas that have been optimized empirically. One novel approach that allows better IOL prediction is to use intraoperative OCT measurements of the aphakic eye under standardized conditions. During surgery, the distance between the anterior lens capsule (in the aphakic eye) and the cornea is measured with OCT (Figure 2). The underlying concept is that the position of the aphakic anterior lens capsule better predicts the IOL position than preoperative biometric parameters.6,7 I believe that this technology, which is still in the prototype stage, will result in significantly better refractive outcomes, especially in short eyes.
Oliver Findl, MD, MBA, FEBO, is Director and Professor of Ophthalmology at the Hanusch Hospital, Vienna, Austria, and a Consultant Ophthalmic Surgeon at Moorfields Eye Hospital, London. He is the Founder and Head of the Vienna Institute of Research in Ocular Surgery (VIROS), Hanusch Hospital, Department of Ophthalmology, Vienna, Austria. Dr. Findl states that he has no financial interest in the instruments or techniques he describes. He may be reached at e-mail: oliver@findl.at.
DETLEF HOLLAND, MD
Achieving good functional outcomes after refractive cataract surgery is the biggest challenge for surgeons, as these have the largest impact on patient confidence postoperatively. Therefore, I believe that the patient work-up is the key step to surgery.
It is important to first understand a patient’s expectations before deciding which procedure and IOL are the most suitable. In patients with high visual demands at all distances including excellent intermediate visual acuity, for instance, I suggest the AT LISA tri IOL (Carl Zeiss Meditec). Alternatively, in patients who demand spectacle independence for most tasks, tend to drive at night, and have no problem wearing glasses for extended reading, I discuss a multifocal IOL with reduced near addition, such as the Mplus Comfort (Oculentis).
To exclude any ocular pathology, I use a wide range of diagnostic exams. Old-fashioned dry eye exams such as tear break-up time and Schirmer testing are important, as dissatisfaction after multifocal IOL implantation in elderly patients often derives from ocular surface problems. Binocular disorders are excluded by a full strabismus examination. Subjective refraction and autorefractometer results are always compared against the existing prescription and the patient’s refractive history.
Pupillometry in scotopic and photopic conditions and dynamic measurements are also performed to exclude patients with abnormally wide pupils that may lead to night driving problems.
All patients undergo corneal topography, corneal and ocular wavefront analysis, and biometry with the IOLMaster. All of these data must be compared in order to achieve the most reliable information; it is especially important to obtain accurate measurements for the amount and axis of astigmatism, and to do this an average of all resulting data must be determined.
As in the field of corneal refractive surgery, comparing the ocular wavefront analysis with the corneal wavefront allows the surgeon to decide whether a customized treatment is necessary (eg, whether a toric multifocal IOL will be the best solution). I prefer toric IOLs in all patients with 0.75 D or more of regular astigmatism. Patients with HOAs should be excluded from refractive lens exchange because these aberrations can lead to decreased visual quality. All of these measurements should be performed on two different dates, if possible, in order to note deviations.
My highest priority in cataract surgery patients is obtaining an OCT of the macula. Very often this test detects pathologies such as vitreous traction or the beginnings of macular degeneration that were not seen on retinoscopy. In the presence of any macular pathology, I do not implant a multifocal IOL.
All patients are informed about possible problems such as residual refractive error and the potential need for lens exchange or laser touch-up; however, by using the approaches outlined above, I have achieved good results and high patient satisfaction in the majority of patients.
Detlef Holland, MD, is a cataract and refractive surgeon at the Augenklinik Bellevue in Kiel, Germany. Dr. Holland states that he has no financial interest in the instruments or techniques he describes. He may be reached at e-mail: d.holland@augenklinik-bellevue.de.
ERIK L. MERTENS, MD, FEBOphth
I find that the following components of the preoperative assessment are important in refractive cataract surgery:
-
K measurements with the IOLMaster, provided the tear film quality is good and stable. These must be compared with corneal topography to confirm that the cornea has astigmatism and to assess whether the astigmatism is regular;
-
Detailed measurement of axial length with the IOLMaster or equivalent;
-
Use of modern formulas to calculate the IOL power;
-
Use of sophisticated instruments such as Optovue OCT to calculate the IOL power in postrefractive surgery cases; and
-
OCT to establish the quality of the retina and macula.
The quality of the tear film and the ocular surface should be optimized before any of these diagnostic exams or surgery is performed.
Erik L. Mertens, MD, FEBOphth, is Medical Director of Medipolis, Antwerp, Belgium. Dr. Mertens is a Co-Chief Medical Editor of CRST Europe. He states that he has no financial interest in the instruments or techniques he describes. He may be reached at tel: +32 3 828 29 49; e-mail: e.mertens@medipolis.be.
BOJAN PAJIC, MD, PhD, FEBO
We have entered a time in cataract surgery when patients expect precise refractive results and maximum visual quality postoperatively. In order to detect potential corneal pathologies before surgery, it is essential to perform the same screening that is required before refractive surgery, including optical biometry, corneal topography, and anterior segment OCT.
[Courtesy of Bojan Pajic, MD, PhD, FEBO]
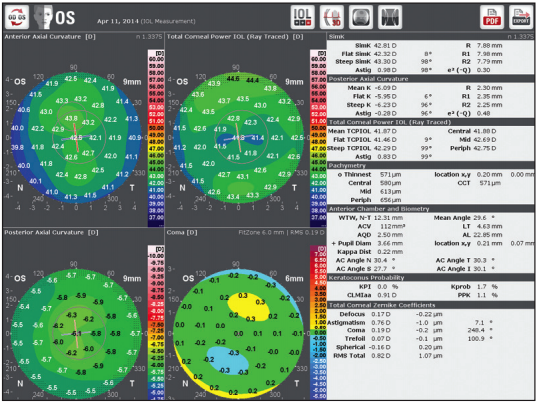
Figure 3. Galilei can aid in precise cataract surgery planning.
In my clinic workflow, I use the Galilei G6 Lens Professional (Ziemer Ophthalmic Systems), a combination of a dual-Scheimpflug analyzer, a Placido-disc system, and an optical biometer. This single machine delivers all the information I need for precise cataract surgery planning (Figure 3).
In one measurement session pre- and postoperatively, this device provides me with a comprehensive dataset of total corneal astigmatism and HOAs as well as precise pachymetry and high-definition elevation maps to plan arcuate incisions. Combined with axial length information, anterior chamber depth, and K readings, also generated by the Galilei, I have all the data needed to implant premium IOLs.
For the exact placement of toric IOLs and in preoperative screening for keratoconus or ectasia, information on both the anterior and posterior corneal surfaces is needed. I therefore always have a close look at posterior corneal curvature and elevation and total corneal astigmatism to detect asymmetries and any corneal bulging. Scheimpflug devices enable us to measure the posterior surface of the cornea instead of making mathematical approximations.
Bojan Pajic, MD, PhD, FEBO, is a Consultant in the Division of Ophthalmology, Department of Clinical Neurosciences, University Hospitals of Geneva, Switzerland; the Medical Director of the Swiss Eye Research Foundation, Eye Clinic Orasis, Reinach, Switzerland; a member of the medical faculty, Military Medical Academy, University of Defense, Belgrade, Serbia; and a surgeon and member of the Board of Specialized Hospital Vidar-Orasis Swiss at the Eye Hospital Vidar-Orasis Swiss, University of Novi Sad, Faculty of Physics, Novi Sad, Serbia. Dr. Pajic states that he has no financial interest in the instruments or techniques he describes. He may be reached at e-mail: bpajic@datacomm. ch.
Drexler WL, Findl O, Menapace R, et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol. 1998;126(4):524-534.
Findl O, Drexler W, Menapace R, et al. High precision biometry of pseudophakic eyes using partial coherence interferometry. J Cataract Refract Surg. 1998;24(8):1087-1093.
Hirnschall N, Murphy S, Pimenides D, et al. Assessment of a new averaging algorithm to increase the sensitivity of axial eye length measurement with optical biometry in eyes with dense cataract. J Cataract Refract Surg. 2011;37(1):45-49.
Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34(3):368-376.
Hirnschall N, Hoffmann PC, Draschl P, et al. Evaluation of factors influencing the remaining astigmatism after toric intraocular lens implantation. J Refract Surg. 2014;6:1-7.
Hirnschall N, Norrby S, Weber M, et al. Using continuous intraoperative optical coherence tomography measurements of the aphakic eye for intraocular lens power calculation [published online ahead of print February 11, 2014]. Br J Ophthalmol. 2014. doi: 10.1136/bjophthalmol-2013-304731.
Hirnschall N, Amir-Asgari S, Maedel S, et al. Predicting the postoperative intraocular lens position using continuous intraoperative optical coherence tomography measurements. Invest Ophthalmol Vis Sci. 2013;54(8):5196-5203.
