As pseudophakic IOL designs evolve, the holy grail of lens-based refractive surgery remains the development of a truly accommodating IOL—one that produces a dynamic change in the dioptric power of the eye when the patient refocuses from distant to intermediate or near objects. A number of shape-changing accommodating IOL designs have been developed and are in various stages of evaluation.1
Even if short-term success is demonstrated, however, vexing questions remain. Will these IOLs maintain their accommodative amplitude in the long term given the effects of postoperative wound healing on the capsular bag? Is the capsular bag a suitable place for an accommodating IOL, or would ciliary sulcus fixation obviate these concerns? Also, what would be the effect of an Nd:YAG laser capsulotomy on accommodating IOL function? Conversely, after a laser capsulotomy, what impact would an accommodating IOL that had absorbed potential energy from capsular forces have on either IOL stability or uncontrolled expansion of that capsulotomy?
IMPACT OF IOL DESIGN ON CAPSULAR WOUND HEALING
Early accommodating IOLs that did not fill the capsular bag and separate the anterior and posterior capsular leaflets encountered problems associated with capsular contraction syndromes.2 For example, asymmetric capsular contraction forces at times brought the hinged plate-haptic Crystalens accommodating IOL (Bausch + Lomb) into a Z-configuration, with one plate haptic vaulted anteriorly and the other posteriorly. The resulting oblique displacement of the lens optic produced astigmatism along the long axis of the IOL.
Postoperative capsular wound healing follows both fibrotic and regenerative pathways.3 In the former, anterior capsular lens epithelia undergo pseudofibrous metaplasia, causing fibrosis and opacification in areas where the posterior aspect of the anterior capsule meets the IOL optic as well as a fibrotic form of posterior capsular opacification (PCO) and capsular contraction. At times, these processes have caused IOL decentration.
The regenerative component involves the migration of equatorial lens epithelial cells (LECs) along the posterior capsule behind the IOL. LECs regenerate and form lens material and consequent PCO, sometimes associated with lens fiber differentiation in the formation of Elschnig pearls and a Soemmering ring.
In an effort to avoid these adverse events, accommodating IOLs such as the NuLens DynaCurve (NuLens) were designed to be inserted and fixated outside of the capsular bag in the ciliary sulcus, but they were found to have a high rate of PCO and some incidence of IOL tilt or malposition.4
In contrast, cumulative animal and clinical data suggest that certain IOLs and endocapsular devices that expand and fill the capsular bag5-8 such as Juvene (LensGen), FluidVision (Alcon), Synchrony (Johnson & Johnson Vision), Zephyr (Anew Optics), and Gemini Refractive Capsule (Omega Ophthalmics) significantly limit PCO and Elschnig pearl development, with only a small degree of Soemmering ring formation observed. The element common to these devices is that they fill the capsular bag and separate the anterior and posterior capsular leaflets, thereby minimizing the equatorial zippering effect that leads to LEC migration and proliferation.
Accommodating IOL designs that keep the anterior capsular edge open and extended may also allow LEC egress from the bag into the aqueous (Figure 1). This process may elicit some low-grade anterior chamber inflammation, as noted for 3 months in the LensGen Grail study, requiring gradual, extended tapering of topical steroids to control the inflammation (data on file with LensGen).
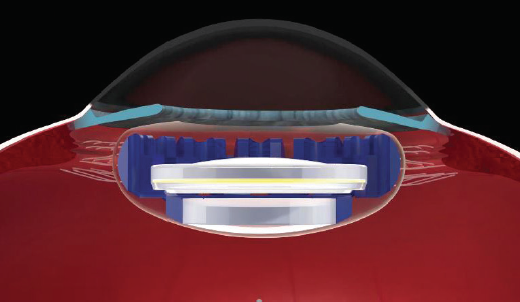
Figure 1. The Juvene accommodating IOL has several features that may mitigate postoperative capsular fibrosis and opacification. The base lens fills the capsular bag, separating the anterior and posterior capsular leaflets and contributing compression force to the posterior capsule. The anterior lens capsule has minimal contact with the lens optic, also allowing the egress of LECs into the aqueous. Finally, fenestrations within the IOL facilitate intracapsular aqueous flow.
It has been hypothesized that certain accommodating IOL designs with fenestrations or holes that serve as channels to facilitate the intracapsular irrigation of aqueous humor may also limit LEC transformation and subsequent proliferation, thus minimizing PCO and fibrotic changes between IOL components. The proposed mechanism is as follows. Enhanced aqueous flow dilutes the concentration of proliferative cytokines bathing lens epithelia to below threshold levels that trigger proliferation8 and expose the lens epithelia to certain growth factors such as transforming growth factor-beta 2 that have been shown to inhibit LEC proliferation.9
Devices that mechanically compress the capsular bag may also have an antiproliferative effect on LECs.8
SLIT-LAMP OBSERVATIONS OF THE POSTERIOR CAPSULE AFTER ACCOMMODATING IOL IMPLANTATION
In the LensGen Grail study of 61 patients observed for 12 to 24 months, trace PCO was noted in one patient on day 1, four patients at month 1, and one patient at both months 3 and 6. In six of the seven participants in whom mild PCO was noted, however, PCO was reported at sequential visits in only one, and none was observed at the 12- or 24-month visit.
Similarly, in a pilot study of an earlier version of the Juvene accommodating IOL involving three patients at 24 months, four patients at 36 months, and four patients at 48 months, no PCO or fibrosis was noted in any of them (data on file with LensGen). Nor has an Nd:YAG laser capsulotomy been required for PCO in either these patients or those in the Grail study.
REFRACTIVE STABILITY AND LONG-TERM ACCOMMODATION FINDINGS
As demonstrated in Figure 2, the monocular defocus curves for the Juvene accommodating IOL are nearly identical at 1 and 12 months, with a similar percentage of individuals achieving distance-corrected near visual acuity of 20/40 or better at 40 cm. Additionally, one patient at 48 months, three patients at 36 months, and two patients at 24 months who received an earlier prototype in a pilot study experienced no decrease in distance-corrected intermediate or distance-corrected near visual acuity (data on file with LensGen).
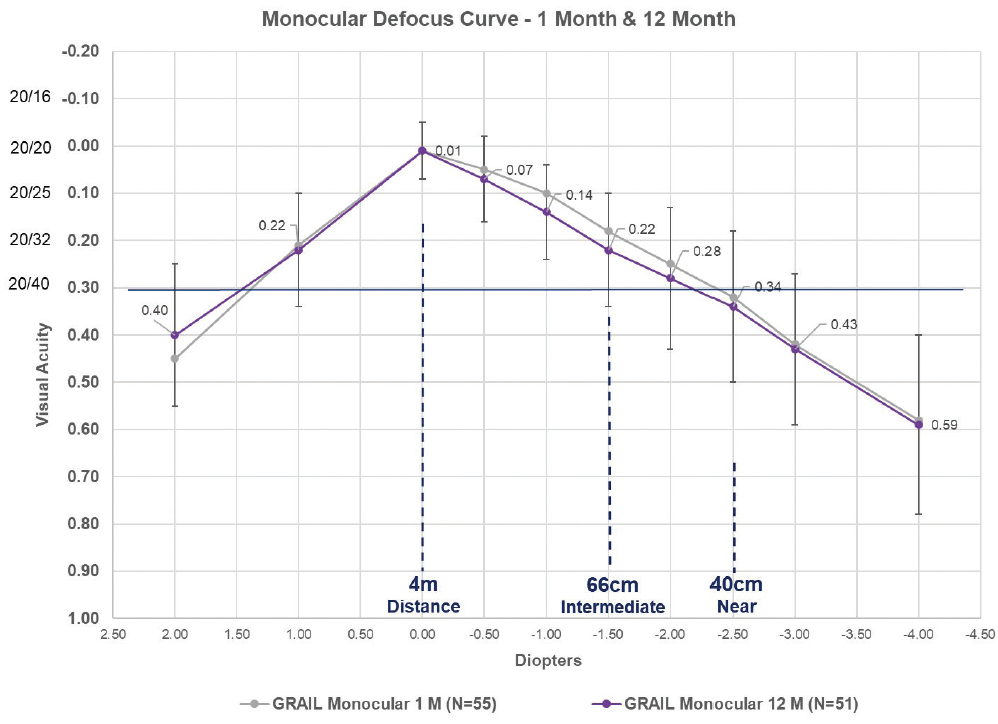
Figure 2. The monocular defocus curves at 1 and 12 months of patients who received the Juvene accommodating IOL in the LensGen Grail study. At zero defocus, mean best corrected distance visual acuity was 20/20. At -1.50 D defocus (representative of intermediate vision), mean distance-corrected intermediate visual acuity was 20/32. At -2.50 D defocus (representative of near vision), mean distance-corrected near visual acuity was between 20/40 and 20/50, with 79% (42/53) of eyes seeing 20/40 or better at 1 month and 82% (42/51) of eyes seeing 20/40 or better at 12 months.
REMAINING CHALLENGES
With any new technology that represents a true paradigm shift, there are some existing challenges and others that will likely arise after larger numbers of patients undergo lens implantation and long-term follow-up. Several questions have yet to be answered fully.
In cataract surgery patients undergoing standard monofocal IOL implantation in which the capsular leaflets are not extended, studies using capsular sizing rings show approximately 13% contraction of the capsular bag during the first 2 months postoperatively.10-14 Will some degree of capsular contraction occur even when an accommodating IOL separates the capsular leaflets and fills the bag? If so, what impact will this have on IOL function and sizing?
Will sizing of the accommodating IOL diameter be critical? If too large an accommodating IOL is inserted, could this produce undue compressive forces that leave the IOL in a pseudoaccommodative stage, leading to a myopic outcome with limited dynamic range of accommodative amplitude? Studies using capsular tension rings and modified capsular measuring rings have demonstrated a statistically significant correlation between axial length and capsular bag diameter as well as other biometric factors.12,13 The correlation between axial length and capsular bag size is fairly robust for axial lengths up to about 25 mm, whereas there are more outliers in this relationship with longer eyes. What are the best method and metric to accurately size an accommodating IOL?
If PCO develops, how can an Nd:YAG laser capsulotomy be performed safely without unduly expanding the opening, and what is the potential impact of a laser capsulotomy on accommodation?
Finally, what will outcomes beyond 5 years be? Early clinical data look promising. Time will tell, however, if accommodating IOLs can meet the long-term challenges imposed by the capsular bag.
1. Pepose JS, Burke J, Qazi M. Accommodating intraocular lenses. Asia Pac J Ophthalmol (Phila). 2017;6(4):350-357.
2. Pepose JS, Burke J, Qazi MA. Benefits and barriers of accommodating intraocular lenses. Curr Opin Ophthalmol. 2017;28(1):3-8.
3. Wormstone IM, Eldred JA. Experimental models for posterior capsule opacification research. Exp Eye Res. 2016;142:2-12.
4. Alió JL, Ben-nun J, Rodríguez-Prats JL, Plaza AB. Visual and accommodative outcomes 1 year after implantation of an accommodating intraocular lens based on a new concept. J Cataract Refract Surg. 2009;35(10):1671-1678.
5. Floyd AM, Werner L, Liu E, et al. Capsular bag opacification with a new accommodating intraocular lens. J Cataract Refract Surg. 2013;39(9):1415-1420.
6. Marques EF, Castanheira-Dinis A. Clinical performance of a new aspheric dual-optic accommodating intraocular lens. Clin Ophthalmol. 2014;8:2289-2295.
7. Bontu S, Werner L, Kennedy S, et al. Long-term uveal and capsular biocompatibility of a new fluid-filled, modular accommodating intraocular lens. Published online August 14, 2020. J Cataract Refract Surg. doi: 10.1097/j.jcrs.000000000000039.
8. Kramer GD, Werner L, Mamalis N. Prevention of postoperative capsular bag opacification using intraocular lenses and endocapsular devices maintaining an open or expanded capsular bag. J Cataract Refract Surg. 2016;42(3):469-484.
9. Kurosaka D, Nagamoto T. Inhibitory effect of TGF-b2 in human aqueous humor on bovine lens epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1994;35(9):3408-3412.
10. Vass C, Menapace R, Schmetterer K, Findl O, Rainer G, Steineck I. Prediction of pseudophakic capsular bag diameter based upon biometric values. J Cataract Refract Surg. 1999;25(10):1376-1381.
11. Dong EY, Joo CK. Predictability for proper capsular tension ring size and intraocular lens size. Korean J Ophthalmol. 2001;15(1):22-26.
12. Kim JH, Lee D, Cha YD, Oh SH, Mah KC, Lee MS. The analysis of predicted capsular bag diameter using modified model of capsule measuring ring in Asians. Clin Exp Ophthalmol. 2008;36(3):238-244.
13. Tehrani M, Dick HB, Krummenauer F, Pfirrmann G, Boyle T, Stoffelns BM. Capsule measuring ring to predict capsular bag diameter and follow its course after foldable intraocular lens implantation. J Cataract Refract Surg. 2003;29(11):2127-2134.
14. Strenn K, Menapace R, Vass C. Capsular bag shrinkage after implantation of an open-loop silicone lens and a poly(methylmethacrylate) capsule tension ring. J Cataract Refract Surg. 1997;23(10):1543-1547.