Epithelium-off (epi-off) CXL was described by a group based in Dresden, Germany, in the early years of this century.1 A system for performing epi-off CXL was approved by the FDA in 2016.2 This procedure is an innovative alternative for the management of keratoconus, but it carries the risks of pain, corneal haze, scarring, and infection. These potential drawbacks spurred us to develop a novel, cost-effective, noninvasive, first-in-class, light-independent pharmacologic alternative—eye drops that induce physiologic CXL.
EARLY RESEARCH
Our efforts were inspired by genetic, biochemical, and tissue evidence of reduced lysyl oxidase (LOX) activity in keratoconic corneas. We developed a topical eye drop (IVMED-80, iVeena Delivery Systems) primarily for the treatment of keratoconus through the upregulation of LOX and thus the induction of endogenous physiologic CXL of corneal collagens. In initial laboratory experiments, IVMED-80 increased LOX activity in both healthy and keratoconic corneal fibroblasts.
In 2017, iVeena’s director of research and development, Sarah Molokhia, PhD, started in vivo experiments after IVMED-80 was granted an orphan drug designation by the FDA and funding support was received in the form of a National Institutes of Health Small Business Innovation Research grant. In a rabbit model, administering the drug twice daily for 7 weeks increased the corneal elastic modulus and CXL (as evidenced by higher lysyl norleucine levels) and produced 1.70 D of corneal flattening on topography. The drops were well tolerated, and there was no inflammation, evidence of pain, or systemic organ damage (unpublished data on file with iVeena).
CLINICAL TRIAL RESULTS
In February 2019, we started a phase 1/2a randomized, controlled, double-masked clinical study to evaluate the safety and preliminary efficacy of IVMED-80 for the pharmacologic CXL of keratoconus (Table).
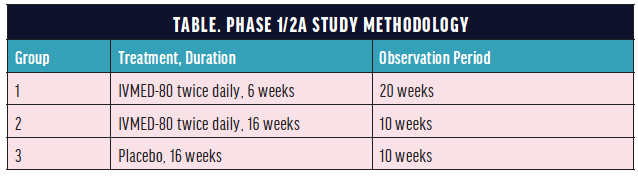
A total of 31 patients with keratoconus, about one-third of the study population, were randomly assigned to each group and completed the observation period during the study, which was approved by the Mexican government’s Federal Commission for the Protection Against Sanitary Risk and was conducted at the Codet Vision Institute in Tijuana, Mexico. The principal investigator of the trial was Arturo Chayet, MD. The experimental design regimen and observation period allowed an assessment of the impact of therapy duration and cessation.
This phase 1/2a study demonstrated that IVMED-80 was safe and well tolerated by patients. No serious ocular or systemic adverse events were reported, including no significant changes in IOP, inflammation, corneal scarring, or endothelial cell count. One patient in the placebo group developed marginal keratitis, which resolved after pharmacologic treatment with no sequelae. Six weeks of IVMED-80 therapy were not sufficient to establish a statistically significant benefit, but the patients in group 2 achieved excellent results, exhibiting a slower progression of keratoconus and a stable corneal flattening effect at 16 weeks that was maintained through the 26 weeks of observation. These results lessened our concern about a regression of corneal flattening after treatment cessation.
There was a statistically significant outcome in primary efficacy with a reduction in longitudinal baseline-adjusted maximum keratometry reading (Kmax) in patients who received IVMED-80 for 16 weeks relative to placebo of 1.00 D (P = .01991). This suggests clinically significant flattening and a high probability of attaining the FDA efficacy benchmark for Kmax reduction (ie, 1.00 D at 1 year). Secondary efficacy variables also showed promising results, including a decline in mean corneal astigmatism in group 2 at 16 weeks and further improvement at 26 weeks. More extensive studies with a longer follow-up period are required to draw conclusions on the possibility of LOX corneal remodeling to enhance corneal symmetry.
Additionally, patients in group 2 achieved a statistically significant gain of 11.3 ETDRS letters from baseline compared with 8.0 letters in group 3. Corneal biomechanics were also positively affected, as evidenced by significant increases in the stiffness parameter highest curvature and stress-strain index, which measures resistance to corneal deformation (Figure). Improvements were comparable to those found after epi-off CXL.
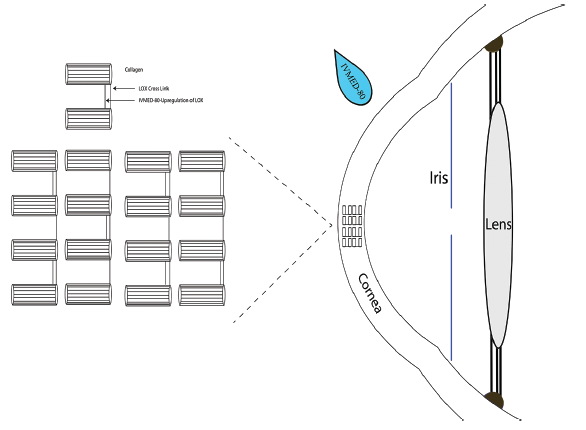
Figure. Corneal strengthening by IVMED-80.
Courtesy of Arun Pughazendhi, MBBS, and Noraliz García-O’Farrill, MD
CONCLUSION
The results of this phase 1/2a study are encouraging. Pending the outcomes of discussions with the FDA, the company’s plan is to pursue further clinical development of IVMED-80 in phase 2b/3 and phase 3 studies. We are excited about the potential of this noninvasive treatment for keratoconus.
1. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620-627.
2. Belin MW, Lim L, Rajpal RK, Hafezi F, Gomes JAP, Cochener B. Corneal Cross-Linking: Current USA Status: Report From the Cornea Society [published correction appears in Cornea. 2019 Oct;38(10):e49]. Cornea. 2018;37(10):1218-1225.