
Several years ago, during a presentation for the Refractive Surgery Alliance (RSA), I described the four stages of vision development throughout life. Around that same time, in 2015, I received the President’s Award from the RSA for best exemplifying the values and mission of the organization. These two accomplishments, I am confident, went hand in hand. I believed then, as I do now, that the aging lens presents an array of opportunities for surgical vision correction and that it is our duty to counsel patients and suggest the procedure for which they are best suited. Most of the time, the best procedure coincides with the patient’s age.
THE FOUR STAGES
Growth phase. The first stage of vision development occurs in childhood and adolescence. The incidence of myopia is increasing worldwide,1 and theories as to why this may be focus mainly on the increased use of handheld devices such as smartphones, increased hours spent studying, and decreased hours spent on outdoor activities.2,3 Recent studies have confirmed what common sense suggests: Myopia can have social and emotional consequences in children that can limit their participation in activities and impair their self-esteem.4 Vision correction surgery is not generally recommended during the growth phase, but treatments can be performed when necessary to provide normal or nearly normal vision and function and even to slow myopic progression.
Ocular maturity. The second stage of vision development occurs in early adulthood, usually starting by the age of 18. At this stage, quick, safe, and comfortable vision correction procedures such as LASIK, PRK, SMILE, and phakic IOLs are safe and effective. In the absence of underlying conditions, the effects of these procedures can last for a lifetime; however, patients will ultimately need other corrective measures, such as cataract surgery.
Presbyopia. Patients enter the third stage of vision development when they develop dysfunctional lens syndrome (DLS), which occurs between 40 and 50 years of age. DLS is marked by the loss of reading vision. Treatments ranging from LASIK to corneal inlays to lens-based surgery are available. Results can be permanent in the absence of other conditions.
Cataract. The final stage of vision development is advanced DLS with cataract formation, and it occurs in individuals aged 50 years and older. People in this age range often think they are not candidates for refractive surgery, but in fact many are excellent candidates for a premium IOL.
PHAKIC IOLS
Some studies have shown that, compared with LASIK and PRK, phakic IOL surgery provides better BCVA and refractive predictability, greater refractive stability, and higher patient satisfaction. Phakic IOL surgery also avoids the risks of postoperative corneal ectasia5 and of retinal detachment associated with refractive lens exchange.
Both anterior and posterior chamber phakic IOLs are available today. The ArtiLens (Ophtec), an anterior chamber phakic IOL, is fixated to the iris. This IOL is first centered in front of the pupil, and then the iris tissue in the midperiphery is enclosed between the claws to hold the implant in place. It is available in both a rigid PMMA model (Figure 1A) and a foldable version called the Artiflex (Figure 1B). The ArtiLens can correct myopia, hyperopia, and astigmatism.
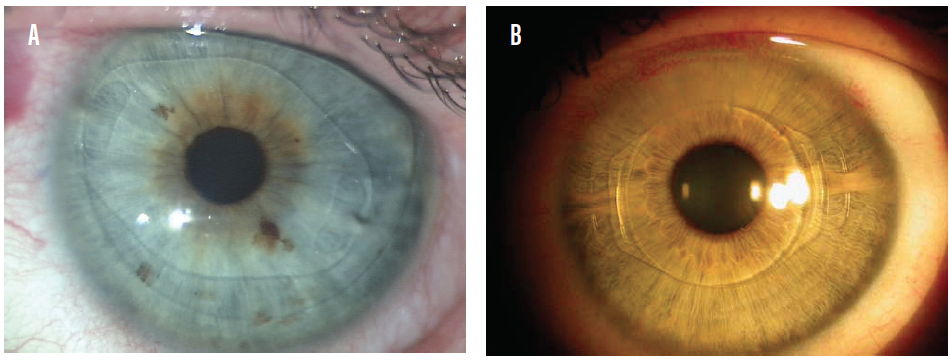
Figure 1. The Artisan (A) and Artiflex (B) phakic IOLs in situ.
Posterior chamber IOLs have gained popularity in recent years as their safety profiles have improved. The EVO Visian ICL (STAAR Surgical) is a one-piece posterior chamber phakic IOL designed with a central port to eliminate the need for an iridotomy or iridectomy. By allowing sufficient aqueous flow from the posterior chamber to the anterior chamber, the central port functions to maintain the normal physiology of the anterior segment (Figure 2).6
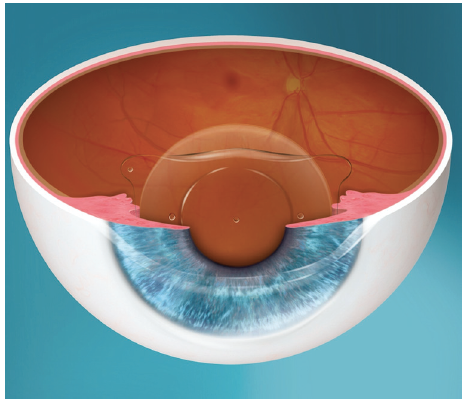
Figure 2. The EVO Visian ICL.
Another model, the EVO+ Visian ICL, has an extended optical zone to accommodate eyes with large pupils, and it is available in powers from -0.50 to -14.00 D (Figure 3). STAAR Surgical has submitted multicenter European pivotal clinical trial data for the EVO+ Visian ICL with an aspheric extended depth of focus optic. Results from the clinical trial were submitted for EU medical device regulation in July 2019. If approved, this lens could become commercially available in countries recognizing the CE Mark in the second quarter of this year.
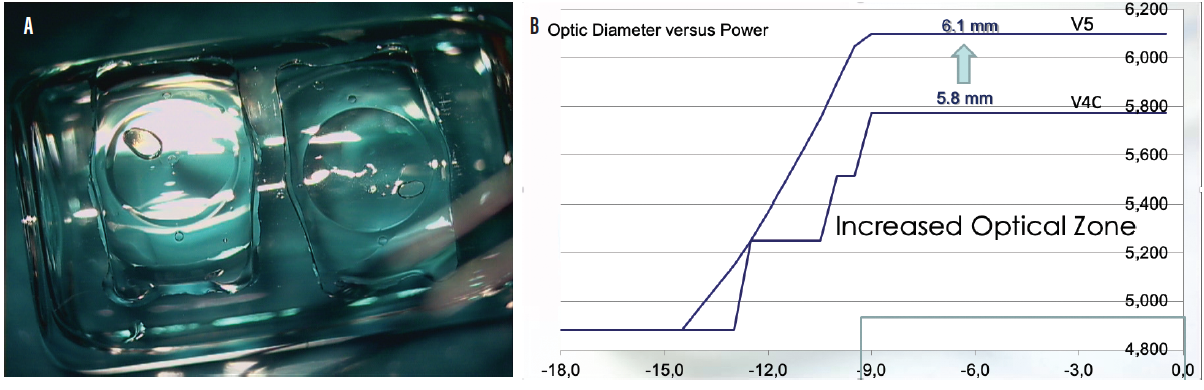
Figure 3. The EVO+ Visian ICL (A) has an extended optical zone (B) to accommodate eyes with larger pupils.
The Implantable Phakic Contact Lens (IPCL V2.0, Care Group), another posterior chamber phakic IOL, has a trifocal diffractive optic (optical zone, 3.5 mm in diameter). This hydrophilic acrylic lens is available with additions ranging from 1.50 to 3.50 D in 0.50 D steps. No peer-reviewed articles have been published on this technology.
PRESBYOPIA-CORRECTING IOLS
IOL technology has evolved in the past decade, and it is our duty to be familiar with the wide range of premium IOLs available today. We surgeons must choose the most appropriate IOL based on our skills and knowledge and on each patient’s lifestyle and specific visual needs. The market has exploded with new IOLs that use different refractive principles. As with any surgical procedure, it is essential to counsel patients properly, set realistic expectations, and take care with patient selection. Patients must understand that they may experience contrast sensitivity loss, glare, and halos after surgery. Surgeons must be prepared to perform precise IOL power calculations and preoperative evaluations and to treat ocular comorbidities such as ocular surface disease and epithelial basement membrane dystrophy. We must also weigh the benefits and drawbacks with each option.
Bifocal diffractive IOLs. Diffractive IOLs such as the Tecnis (Johnson & Johnson Vision) and AcrySof Restor (Alcon) create two distinct images at near and far. The AcrySof Restor platform is based on apodization, in which a near-dominant central area is surrounded by concentric rings of decreasing height that result in the diffraction of light at both distance and near.7,8 Multiple near add powers are available to suit patients’ visual requirements. The Tecnis diffractive IOL has an aspheric anterior surface and a posterior surface with diffractive rings that focus both near and distant light at any pupillary size. Diffractive IOLs are typically less limited by pupillary diameter, but patients can experience poor intermediate vision.9
Segmented bifocal IOLs. The Mplus and Mplus X (both from Oculentis) and the SBL-3 (Lenstec) are rotationally asymmetric, with the shape of the near vision segment designed to provide a seamless transition between the near and far vision zones.10
Trifocal diffractive IOLs. In 2010, PhysIOL released the first trifocal IOL, the FineVision (Figure 4).11 Thereafter, other trifocal IOLs, including the AT LISA (Carl Zeiss Meditec), PanOptix (Alcon), Trinova (VSY), Acriva Reviol (VSY), and Alsafit (Alsanza), have entered the market. Trifocal IOLs typically improve intermediate vision by providing a third point of focus. Higher-order aberrations and visual quality with trifocals have been shown to be similar to those of bifocal diffractive IOLs.12,13
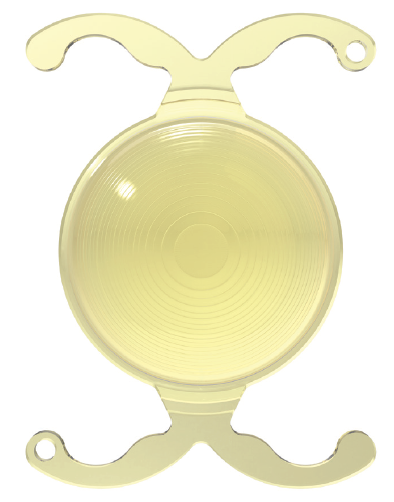
Figure 4. The FineVision IOL.
Extended depth of focus IOLs. Whereas multifocal IOLs have two or three distinct points of focus with blurry vision in between, extended depth of focus (EDOF) IOLs are designed to elongate the focus of vision without compromising distance visual acuity. Each works on one of the three principles described here:
- Echelette design. The Tecnis Symfony IOL (Johnson & Johnson Vision) has a biconvex anterior aspheric surface and a posterior achromatic diffractive surface with an echelette design that helps to reduce chromatic aberration.14 Postoperatively, patients can show minus values on manifest refraction and at the autorefractor. A fogging technique and high-plus reading glasses can be helpful.
- Low-add multifocal IOLs. Whether these lenses should be classified as an EDOF technology is debatable. They mainly improve distance and intermediate vision, but they split light into two or more points of focus, which reduces contrast sensitivity.15
- Small-aperture IOLs. The IC-8 (AcuFocus, Figure 5) utilizes the pinhole principle to increase depth of focus to about 3.00 D. Of the EDOF IOLs, the IC-8 is most forgiving when the target refraction is missed.16 This IOL is especially effective in patients who have a history of radial keratotomy and in post-LASIK eyes with high corneal irregular astigmatism.
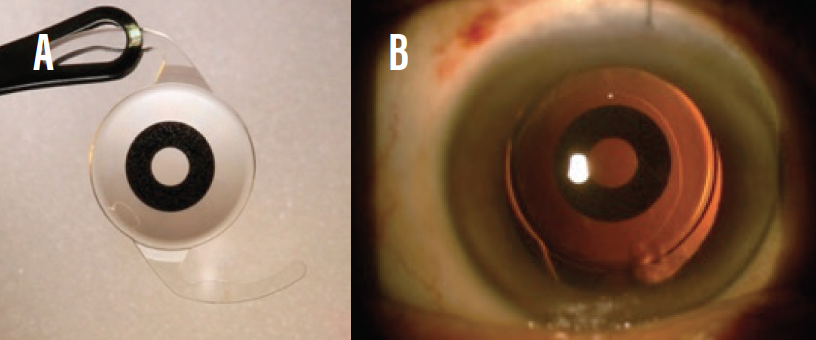
Figure 5. The IC-8 IOL (A); the lens in situ (B).
Hybrid IOLs. Some novel IOLs combine manipulation of aberrations with multifocality. One such lens is the FineVision Triumf (PhysIOL, Figure 6), the first EDOF trifocal IOL. Another example is the Artis Symbiose (Cristalens, Figure 7), which consists of two complementary profiles, one optimized for near vision and the other for intermediate vision. The Artis Symbiose Mid is implanted in one eye to provide intermediate vision, and the Artis Symbiose Plus is implanted in the other eye to provide near vision. Together, they are designed to provide full-focus vision with phase continuity, ensuring image sharpness from 40 to 90 cm without compromising distance vision. Studies of these IOLs are under way, and results can be expected later this year.
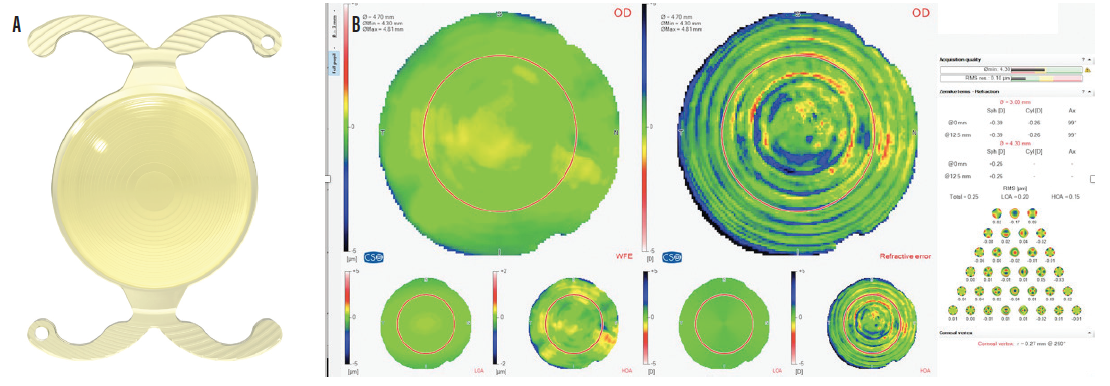
Figure 6. The FineVision Triumf IOL (A); quality of vision with the FineVision Triumf IOL (B).

Figure 7. The Artis Symbiose complementary IOLs.
Accommodating IOLs. Historically, IOLs in this category have simulated the mechanism of action of the human crystalline lens by inducing changes in one of the following: axial position, shape or curvature, or refractive index or power.17 The latest generation of accommodating IOLs uses a variety of mechanisms that rely on ciliary muscle movement or pupillary changes to stimulate and alter the refractive properties of the optic or change its shape.
- The Lumina IOL (AkkoLens International). This lens consists of two optical elements that move one over the other to change the dioptric power of the system.
- The Dynacurve (NuLens). When the ciliary muscle contracts, this out-of-the-bag IOL transmits the forces to a piston, which causes a gel component of the lens to bulge.
- The FluidVision IOL (PowerVision). Fluid inside the FluidVision IOL (Figure 8) moves between the lens’ haptic and optic components, inducing a change in the optic’s curvature and, as a result, in the optical power of the lens.
- The Sapphire IOL (Elenza). This autofocus IOL is electronically controlled by nanotechnology. Pupillary responses stimulate changes in the lens that alter its refraction. The IOL has a power cell that requires recharging every 3 to 4 hours.
- The Juvene (LensGen). During accommodation, capsular forces cause the curvature of this IOL to change. The bulkiness of the Juvene can make it difficult to inject through a small incision.

Figure 8. The FluidVision IOL.
Further research must demonstrate the stability and effectiveness of each of these novel accommodating IOLs. Several are awaiting clinical trials.
CONCLUSION
Phakic IOLs are an increasingly popular treatment option for patients with ametropia, particularly those who are not candidates for laser vision correction. Because the crystalline lens is not extracted, phakic IOL implantation preserves accommodation in young adults searching for independence from glasses and contact lenses. Because no corneal tissue is removed and corneal asphericity is retained, phakic IOL implantation generally does not induce dry eye. Another benefit is that surgery does not affect future IOL calculations.
Presbyopia-correcting IOLs are another effective means of treating ametropia and presbyopia. These lenses help patients to achieve spectacle independence in the majority of cases with a high level of patient satisfaction. The key to success is to closely analyze the expectations of each patient and to match the IOL to his or her lifestyle and visual needs. Proper patient selection, an extensive preoperative evaluation of the ocular surface and macula, accurate IOL power calculations, and careful surgical technique are essential. It is also important to spend adequate chair time with patients. We must explain all of the factors that influence surgical outcomes and possible complications such as blurred vision and photic phenomena, usually due to residual refractive errors, posterior capsular opacification, dry eye, wavefront abnormalities, or IOL decentration. Most of these complications can be managed and may not affect visual outcomes or patient satisfaction and quality of life.
1. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042.
2. Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48(8):3524-3532.
3. Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115(8):1279-1285.
4. Wang D, Yang J, Xian YJ, Wu PP, Lin SL. Current status of social anxiety among primary school students with myopia in Urumqi, China and risk factors for myopia. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21(2):184-188.
5. Barsam A, Allan BD. Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia. Cochrane Database Syst Rev. 2014(6):CD007679.
6. Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;10:1059-1077.
7. Garzón N, García-Montero M, López-Artero E, et al. Influence of angle kappa on visual and refractive outcomes after implantation of a diffractivetrifocal intraocular lens. J Cataract Refract Surg. doi: 10.1097/j.jcrs.0000000000000156. [published online ahead of print February 14, 2020.]
8. Lichtinger A, Rootman DS. Intraocular lenses for presbyopia correction: past, present, and future. Curr Opin Ophthalmol. 2012;23(1):40-46.
9. Montes-Mico R, Ferrer-Blasco T, Charman WN, Cervino A, Alfonso JF, Fernandez-Vega L. Optical quality of the eye after lens replacement with a pseudoaccommodating intraocular lens. J Cataract Refract Surg. 2008;34(5):763-768.
10. Alio JL, Pinero DP, Plaza-Puche AB, Chan MJ. Visual outcomes and optical performance of a monofocal intraocular lens and a new-generation multifocal intraocular lens. J Cataract Refract Surg. 2011;37(2):241-250.
11. Yoon CH, Shin IS, Kim MK. Trifocal versus bifocal diffractive intraocular lens implantation after cataract surgery or refractive lens exchange: a metaanalysis. J Korean Med Sci. 2018;33(44):e275.
12. Rosen E, Alió JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal
intraocular lenses following cataract and refractive lens exchange: metaanalysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42:310-328.
13. Pedrotti E, Carones F, Aiello F, et al. Comparative analysis of visual outcomes with 4 intraocular lenses: monofocal, multifocal, and extended range of vision. J Cataract Refract Surg. 2018;44(2):156-167.
14. Rocha KM. Extended depth of focus IOLs: the next chapter in refractive technology? J Refract Surg. 2017;33(3):146-149.
15. Tarib I, Diakonis VF, Breyer D, Höhn F, Hahn U, Kretz FTA. Outcomes of combining a trifocal and a low-addition bifocal intraocular lens in patients seeking spectacle independence at all distances. J Cataract Refract Surg. 2019;45(5):620-629.
16. Dick HB, Piovella M, Vukich J, Vilupuru S, Lin L, Mertens E. Prospective multicenter trial of a small-aperture intraocular lens in cataract surgery. J Cataract Refract Surg. 2017;43:956-968.
17. You-Ling L, Song-Bai J. Clinical application of accommodating intraocular lens. Int J Ophthalmol. 2018;11(6):1028-1037.



