

Whether we like it or not, we are all going to face a situation like the following. We enter the OR anticipating a routine surgery. To our surprise, a complication occurs. Suddenly, our 10-minute case turns into a 40-minute case, or even longer.
In these situations, the key to surgical success and a satisfactory postoperative outcome is how the case is managed. This articles suggests some tips for handling these unexpected intraoperative challenges (see Tips For Success in Brief).
Tips For Success in Brief
1. Stay calm; do not suddenly withdraw instruments from the eye;
2. Assess the nature and extent of the complication;
3. Try to limit further iatrogenic damage in the eye;
4. Manage the complication or refer to a colleague in case of lack of expertise or experience;
5. Have a disaster preparedness kit in the OR, and train staff on procedures;
6. Keep the patient comfortable;
7. Do the best, most scientific thing in the given situation; and
8. Monitor the patient closely in the postoperative period.
Inject OVD
The surgeon’s first reaction should be to stay calm and assess the situation. We often recommend taking a pause and reflecting on the best way to deal with the situation. The main goal in a complication is to salvage the eye and prevent further damage, which can be iatrogenic. Often, the first reflex of the surgeon in an unanticipated complication is to panic and withdraw the instruments out of the eye. This is exactly what you should not do. OVD should be injected into the eye before any instrument is withdrawn, particularly when a posterior capsule rupture (PCR) or zonular dialysis is suspected.
Assess the Situation AND TRY TO LIMIT DAMAGE
After injecting OVD, calmly assess the extent of damage. To help elucidate our pearls for managing unexpected complications in the OR, let us use a hypothetical scenario in which a PCR occurs.
One of the most dreaded complications is the occurrence of a PCR, with or without the dropping of lens material into the vitreous cavity. In this situation, there are two problems that the surgeon needs to deal with: (1) vitreous presenting in the anterior chamber and (2) residual lens material in the anterior and/or posterior segment. We find the intracameral injection of preservative-free triamcinolone acetonide (4 mg/0.1 mL) useful to help detect the presence of vitreous strands in the anterior chamber. It is important that, if there is vitreous in the anterior chamber, a good anterior vitrectomy be performed before any other maneuvers in the eye.
Manage or Refer
Manipulations such as levitation of nuclear fragments or inserting the phaco probe into the vitreous cavity should be strictly avoided. Any kind of mechanical or fluidic turbulence in the presence of vitreous strands can lead to clinically invisible traction on the vitreous attachments, especially at the vitreous base and the macula, leading to higher chances of retinal detachments, giant retinal tears, or macular edema. Therefore, if nuclear fragments have dropped into the vitreous cavity, instead of chasing them, the surgeon should perform a thorough anterior vitrectomy, clear the residual nuclear or cortical matter with low fluidic parameters, and place an IOL only if it can be placed safely in the capsular bag or ciliary sulcus. Thereafter, a retina surgeon can be engaged to deal with the dropped nuclear material. This can be done either at the same sitting or in a subsequent procedure, depending on the availability of the vitreoretinal surgeon.
When residual nuclear or cortical material is left behind in the anterior segment, the surgeon should not go back to removing the lens until after the vitrectomy has been performed. Phacoemulsification or cortical aspiration can then be performed as routine, although the surgeon must keep in mind to lower the fluidic parameters. That is, bottle height and aspiration flow rate should be lessened so as to avoid further vitreous hydration and turbulence. Principles of slow-motion phacoemulsification, as advocated and described by Robert H. Osher, MD, should be followed.1
From Routine to Complicated
Sneaky Weak Zonules
By D. Brian Kim, MD

Most cataract cases proceed routinely. Occasionally, however, they can go sideways, requiring quick recognition, decisive decision-making, and careful execution to avert disaster. The case presented in the accompanying Eyetube video below provides a close look at zonulopathy and points out the warning signs interwoven throughout the procedure that can tip off the surgeon to potential trouble ahead. This article includes pearls for identifying and tackling eyes with zonulopathy and minimizing further zonular trauma.
PEARL NO. 1
In any patient with a small or medium-sized pupil, the surgeon should suspect zonular disease. If the pupil does not respond to intracameral epinephrine, the patient may have weak zonules, not intraoperative floppy iris syndrome.
PEARL NO. 2
If puncturing the capsule to initiate the capsulorhexis causes capsular striae (Figure 1), this is a telltale sign of zonular weakness. I prefer to use forceps for this step rather than a cystotome for a number of reasons. In this case, this approach was particularly advantageous because I was able to see capsular striae as I was trying to puncture the capsule with forceps, confirming the presence of weak zonules.
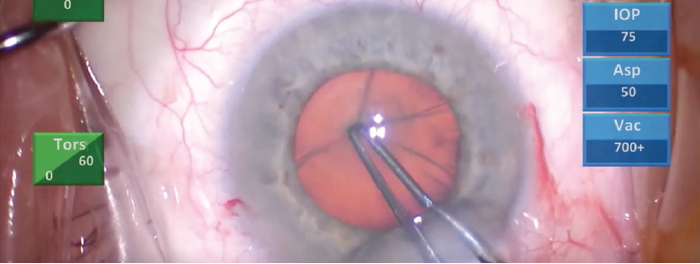
Figure 1. Capsular striae presented when the capsule was punctured to initiate the capsulorhexis, indicating zonular weakness.
The zonules are like the springs of a trampoline. They are supposed to apply outward centrifugal forces to keep the central mesh taut. If the springs are weak, the mesh sags and cannot resist an external force. Similarly weak zonules cannot resist the force of the capsulorhexis forceps. As the forceps push down on the anterior capsule, it is difficult to puncture the capsule, and striae result.
PEARL NO. 3
Capsular striae at the edge of the capsulorhexis flap are additional signs of zonular weakness (Figure 2). The surgeon finds that it is difficult to pull the flap due to poor counterforce from the weak zonules.
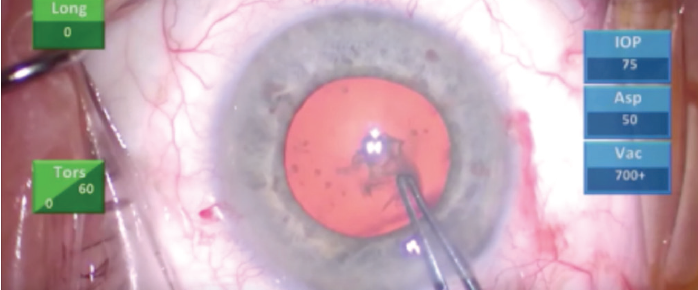
Figure 2. Capsular striae appeared at the edge of the capsulorhexis flap.
PEARL NO. 4
Difficulty spinning the lens after hydrodissection may also be a sign of zonular weakness. When an attempt is made to spin the lens, there are persistent adhesions between the lens and the capsular bag. Normally, the zonules are strong enough to hold the bag in place while the lens is spun with torsional force. When the zonules are weak, however, the adjacent capsular bag tends to stick to the lens, making it difficult to spin.
PEARL NO. 5
When we encounter weak zonules, it is prudent to use a cataract disassembly technique that causes minimal zonular trauma. Mechanical fracturing techniques such as the double chop1 and cross chop2 can be used to disassemble the lens without spinning it, which helps to minimize zonular stress.
PEARL NO. 6
In an eye with weak zonules, do not try to grab the lens pieces with the phaco tip. The phaco tip uses vacuum to grab and hold the lens pieces, and this can be dangerous in the setting of weak zonules. In trying to grab a lens piece, you could grab the capsular bag instead and rip more zonules or cause a capsular rupture. It is far better and safer to use the chopper to pull the lens pieces out of the capsular bag and into the central safe zone (Figure 3).
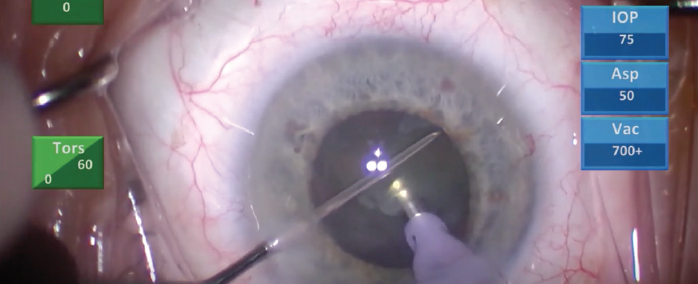
Figure 3. To avoid grabbing the capsular bag by mistake, potentially ripping more zonules or causing a capsular rupture, the chopper can be used to pull the lens pieces into the central safe zone for emulsification.
PEARL NO. 7
Stubborn cortical remnants within the capsular fornix are best liberated by flushing the fornix with pulses of balanced saline solution on a syringe. This frees the cortical material atraumatically. Care must also be taken to flush within the bag, as flushing outside of the bag can cause focal loss of iris pigment.
PEARL NO. 8
When you inject a capsular tension ring, use a Sinskey hook to capture the leading eyelet. As the capsular tension ring is advanced, the Sinskey hook minimizes rotational stress on the zonules.
CONCLUSION
It is my strong opinion that all surgeons should utilize these pearls not only in eyes with weak zonules but in all cataract cases. Employing these techniques will ensure minimal stress on the zonules and at the same time help prepare the surgeon for any potential zonular complication.
1. Kim DB. Cross chop: modified rotationless horizonal chop technique for weak zonules. J Cataract Refract Surg. 2009;35:1335-1337.
2. Kim DB. Double-chop: modified-chop technique eliminating ultrasonic energy and vacuum for lens fragmentation. J Cataract Refract Surg. 2016;42:1402-1407.
From Routine to Complicated
Anterior Capsular Tear
By Maria S. Romero, MD

An anterior capsular tear can be an unwelcome complication at the beginning of a case, requiring the surgeon to rethink his or her approach to the rest of the surgery to ensure a satisfactory outcome. Such a case is shown in the accompanying Eyetube video (bit.ly/0320Romero). The anterior capsular tear occurred during the capsulorhexis, extending radially 180°, beyond the limits of the pupil margin. I didn’t see any signs of posterior capsular compromise at that time; the tear’s extension appeared to be still pre-equatorial.
Because the large tear decreased the oppositional tension required to complete a curvilinear capsulorhexis, I decided to complete the capsulotomy using a can-opener technique (Figure 1), after filling the anterior chamber with OVD.
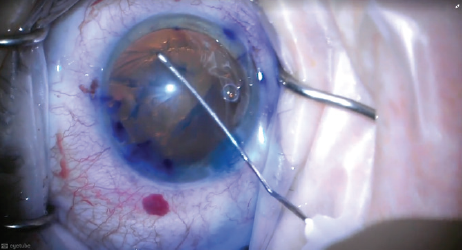
Figure 1. After an anterior capsular tear occurred during capsulorhexis, the surgeon completed the capsular opening with a can-opener technique.
Once the capsulorhexis was complete, I had to make a decision whether to proceed with phacoemulsification or convert to an extracapsular cataract extraction. Because I observed that the posterior capsule still appeared to be stable, I decided to proceed with phacoemulsification, although using an alternative technique to release the lens from the capsule. That is, in order to prevent the tear from extending posteriorly due to the pressure in the capsular bag, I did not perform hydrodissection, but instead released the lens from its capsular bag adhesions using hydrodelineation (Figure 2).
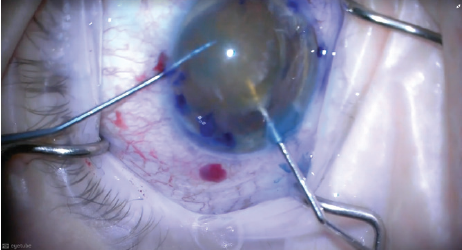
Figure 2. To avoid placing stress on the capsular bag, hydrodelineation was performed rather than hydrodissection to release the lens from the capsule.
With the lens now mobile, I injected an OVD under the lens to partially tilt it into the anterior chamber. Using a bimanual approach with the OVD canula underneath the lens and a chopper on top, I exerted gentle pressure on the lens to slowly move it into the anterior chamber completely (Figure 3).
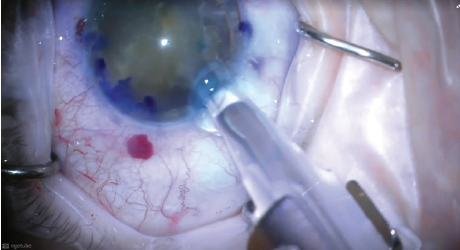
Figure 3. A bimanual approach was employed, with the OVD canula underneath the lens and a chopper on top. Gentle pressure was exerted on the lens to slowly move it into the anterior chamber.
I again coated the endothelium with OVD and then inserted a three-piece IOL behind the lens, aiming the leading optic into the sulcus but leaving the trailing optic outside of the eye. This was a safety step, in the event that the IOL had to be withdrawn due to loss of capsular support at any point (Figure 4).
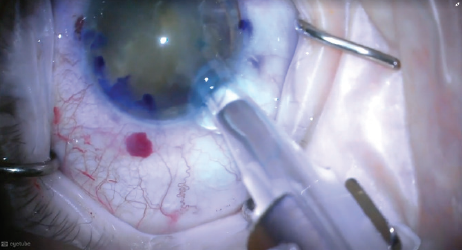
Figure 4. A three-piece IOL was inserted behind the lens, aiming the leading optic into the sulcus but leaving the trailing optic outside the eye.
Once the IOL was implanted, the posterior capsule could act as a platform, separating the anterior and posterior chambers and stabilizing the anterior chamber. I could then completely emulsify the nucleus at the iris plane, still in the anterior chamber (Figure 5).
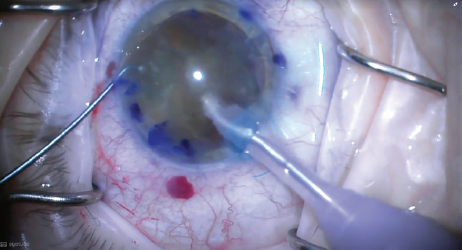
Figure 5. The nucleus was completely emulsified at the iris plane, still in the anterior chamber, with the IOL behind it.
After the lens was completely emulsified and I had a direct view of the posterior capsule, I repositioned the IOL in the bag (Figure 6) and was ready to safely and successfully remove the residual cortical material around and beneath the IOL with irrigation and aspiration.
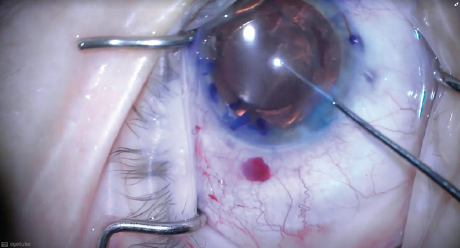
Figure 6. After the lens was completely emulsified, the IOL was repositioned in the bag and the case was completed with cortical irrigation and aspiration.
TWO PATHWAYS FOR VITRECTOMY
Cataract surgeons must understand that the goal of the vitrectomy is to clear the working space of vitreous, not to perform a core vitrectomy. There are two choices at this stage, either a limbal or a pars plana approach to vitrectomy. The pros of a limbal approach are familiarity for the cataract surgeon and the ability to use two corneal paracenteses to perform bimanual vitrectomy. A major disadvantage of performing limbal vitrectomy is that it exerts upward dragging force on the vitreous, which can lead to more prolapse of vitreous and subsequent enlargement of the PCR, in turn jeopardizing in-the-bag placement of an IOL.
By contrast, performing pars plana vitrectomy helps the surgeon deal with the vitreous in its natural compartment. It also pulls the vitreous from the anterior chamber toward the posterior segment, thereby reducing further drag and prolapse of vitreous and preventing further enlargement of the PCR. What’s more, the cataract surgeon can use the corneal paracentesis for the irrigation handpiece, whereas the vitrector is inserted through a pars plana entry made about 3 mm behind the limbus. If the PCR is small, the surgeon can convert the ragged edges of the PCR into a strong, continuous posterior capsulorhexis and place an IOL in the capsular bag.
Irrespective of the approach to vitrectomy, it should be kept in mind that bimanual vitrectomy should always be performed, with infusion through one incision and cutting and aspiration through another. The main incision should never be used because it is larger and does not allow maintenance of a closed anterior chamber during the procedure. If at this time the main incision is found to be leaky, it may be sutured using interrupted sutures.
DISASTER MANAGEMENT KIT
Of crucial importance during complication management is the preparedness of the OR team, including the paramedics and the assistants. A disaster management kit should be available in the OR at all times. It should include devices such as capsular rings, hooks, a dispersive OVD, vitrector, trocars or a microvitreoretinal blade, preservative-free triamcinolone acetonide, and other medications required for contingencies. The OR team should be aware of and experienced with the use of these devices.
Equally important, when the duration of the case extends, is to ensure the comfort of the patient. Often, the surgery may have started with topical anesthesia, and vitrectomy is an unanticipated step. There may be a need to administer supplemental anesthesia, such as sub-Tenon injection, or to add systemic medications with the anesthesiologist’s advice. Postoperatively, it is important to closely monitor and treat any complications such as glaucoma or retinal detachment.
CONCLUSION
To summarize, the mantra for success in case of an unanticipated complication during surgery is to remain calm, assess the extent of damage, and then ensure a quiet, clean eye at the end of surgery. Postoperatively, counseling and frequently evaluating the patient, depending on the requirement, will help surgeons emerge victorious in any challenging situation.
1. Osher RH. Slow motion phacoemulsification approach. J Cataract Refract Surg. 1993;19(5):667.
Mar 2020


