CASE PRESENTATION
A 22-year-old woman presented for laser vision correction (LVC) in 2012. BCVA was 20/15 OU with a manifest refraction of -4.50 D sphere in the right eye and -4.00 D in the left eye. After a 2-week discontinuation of contact lens wear, tomography was significant for mild irregular astigmatism, inferior corneal steepening, and mild posterior float elevation in each eye (Figure 1). The patient exhibited mild dry eye disease and contact lens intolerance but stated that she did not rub her eyes. Her parents and siblings had achieved successful results with LASIK and encouraged her to undergo the procedure. Because of the tomographic findings and her age, we decided to delay surgery and agreed to repeat the measurements in 1 year.
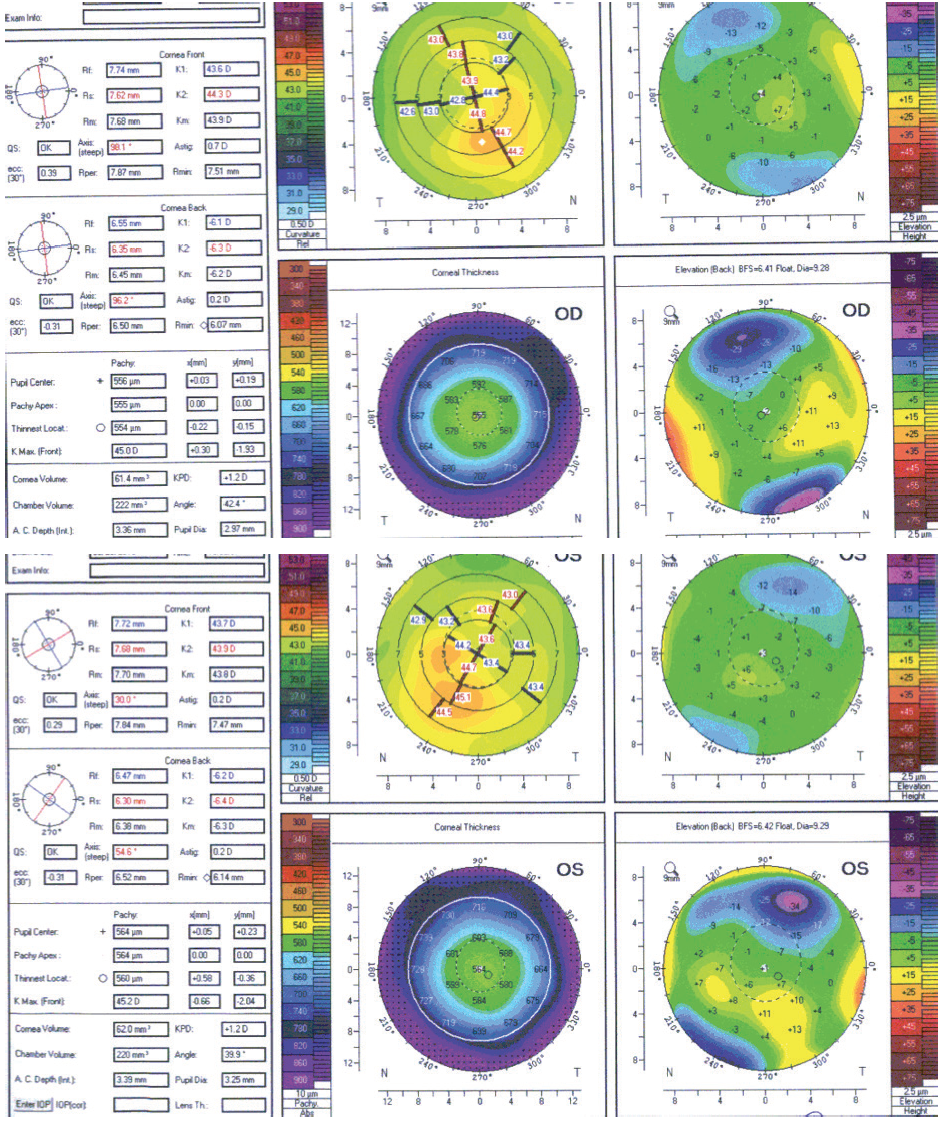
Figure 1. Tomography scans from 2012.
Eight years after her initial consultation, at 30 years of age, the patient returned for an LVC evaluation. Her family members remained satisfied with their LASIK outcomes. The patient continued to experience mild dry eye disease and contact lens intolerance. BCVA was 20/15 OU with a cycloplegic refraction of -4.25 D sphere in the right eye and -3.25 D sphere in the left eye. The average keratometry reading in each eye was 44.00 D. Tomography scans were significant for mild inferior steepening similar in magnitude to what was detected in 2012 (Figure 2). The anterior float had become more regular in each eye. The posterior float and corneal thickness were largely normal in each eye. A biomechanical evaluation using the Keratoconus Match Index on the Ocular Response Analyzer (Reichert) indicated a low suspicion of ectasia in each eye. Of note, the scotopic pupil size was 9 mm in each eye. The patient reported seeing glare and halos at night but said she did not find the phenomena bothersome. She was motivated to undergo LVC.
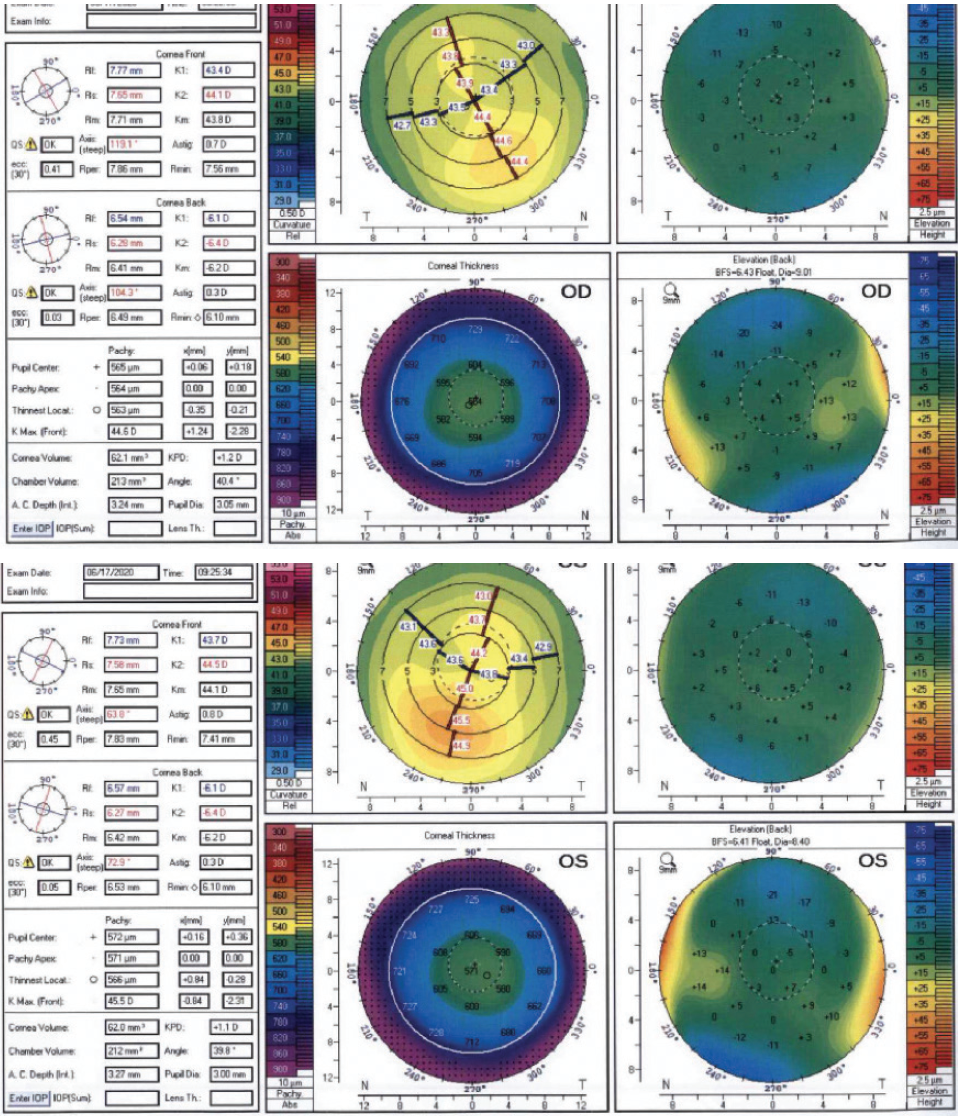
Figure 2. Tomography scans from 2020.
How would you proceed?
—Case prepared by Christopher E. Starr, MD

ARTHUR B. CUMMINGS, MB ChB, FCS(SA), MMed(Ophth), FRCS(Edin)
Given this patient’s level of motivation, the stability of her corneal and pachymetric readings over the course of 8 years, and the absence of risk factors for ectasia (ie, no family history, no eye rubbing, and low biomechanical risk on ORA evaluation), LVC is certainly a possibility. It would be prudent to obtain epithelial maps to exclude an ectatic condition, primarily to inform the patient about her potential need for CXL in the future. My sense is that the epithelial maps will be normal or show epithelial thickening inferiorly (rather than epithelial thinning) and therefore not be indicative of ectasia, which will allow LVC or the implantation of phakic IOLs because she has deep anterior chambers.
The 9-mm scotopic pupils are this patient’s biggest risk factor for dysphotopsia. In my experience, patients similar to this one do not experience glare and halos if treated with a wavefront-optimized excimer laser protocol using an optical zone of 6.5 mm or larger. Based on the excellent pachymetry readings, I would opt for a 7-mm optical zone and enlarge the transition zone to 9 mm. It is important to note that nomogram predictability tends to be slightly worse with this approach than in routine cases using a 6.5-mm optical zone, so this would have to be discussed preoperatively with the patient. She is not currently bothered by glare, so I would not expect her to be bothered by it postoperatively, either, even with a 6.5-mm optical zone.
Topography-guided procedures are known to improve nighttime glare, so this form of LVC could be considered despite the patient’s lack of refractive astigmatism and small amount of topographic astigmatism. The Phorcides Analytic Engine (Phorcides) would be an excellent tool with which to calculate the refraction to be entered into the excimer laser system. LVC should provide excellent results for this patient in terms of refractive outcome. The decision of which form of LVC to perform depends on the epithelial maps. If they show a sign of ectasia, PRK is the best option with signed informed consent noting that the patient should be monitored regularly after LVC and that she may require CXL in the future. If the epithelial thickness maps exclude an ectasia risk, then LASIK and SMILE are options. The bilateral implantation of a Visian ICL (STAAR Surgical) also merits consideration because it is a reversible procedure. The risks and benefits of each strategy should be discussed with the patient before proceeding.

RICHARD S. DAVIDSON, MD
“Guarantees come with refrigerators and freezers, not eye surgery,” said one of my mentors, Ivan Schwab, MD. The concern here is determining the patient’s risk of postoperative corneal ectasia.
I agree that she is at low risk of ectasia, and I like that her refraction has remained stable for 8 years. In addition, although inferior corneal steepening is still evident, her irregular astigmatism looks more regular to me now than on earlier scans. Something to consider is whether eyelid anatomy is contributing to the findings on tomography. For example, the position of the eyelids in some patients can give the appearance of inferior corneal steepening when, in fact, the cornea is completely normal. The total corneal astigmatism is relatively low in this case, which makes me feel more confident about proceeding with LVC.
Steven C. Schallhorn, MD, reported on risk factors for ectasia. In that study involving 180,000 patients, he and his colleagues attempted to determine who would be at highest risk of ectasia. Although significant inferior corneal steepening was associated with an increased risk, many of the patients who developed ectasia did not have what would typically be considered high-risk factors (eg, thin corneas, lower residual stromal beds).1 When counseling this patient, I would explain that her risk of ectasia is low but not zero. I think LASIK is a reasonable option, but if she or her surgeon is very risk averse, PRK or SMILE would carry a lower risk of ectasia.

KATHRYN M. HATCH, MD
This patient has mild, stable-appearing irregular astigmatism and normal corneal pachymetry readings. Given the absence of corneal thinning, a diagnosis of forme fruste keratoconus may have a low likelihood, but it would be helpful to consider other parameters. Specifically, I would review the compare maps feature on the Pentacam (Oculus Optikgeräte) to look carefully for differences between the two evaluations. Additionally, I would review the Belin/Ambrósio D score, which extrapolates anterior and posterior elevations and pachymetric data while accounting for the patient’s age. This score is useful only in a virgin eye but is sensitive for diagnosing subclinical keratoconus. With Placido-based topography, inferior/superior asymmetry, specifically at the 3- and 5-mm zones, can be measured. If the inferior and superior asymmetry observed at the 3- and 5-mm optical zones is less than 1.50 and 2.00 D, respectively, it may also indicate relatively normal indices.
I would also obtain epithelial thickness mapping. If the epithelium has thinned over the steepest areas of the corneas, a diagnosis of forme fruste keratoconus is more likely. I would perform measurements with the Topolyzer Vario (Alcon) to evaluate the patient for higher-order aberrations and to assess her candidacy for a topography-guided treatment. Higher-order aberrations may explain her current nighttime visual symptoms, which a topography-guided treatment has the potential to improve.
Ultimately, the refractive surgeon must make a recommendation to this patient who is now eager to proceed with LVC. Because of her refractive stability for nearly 8 years, it would be reasonable to proceed with LVC after careful informed consent. I would discuss the possibility of performing CXL, but it would be reasonable to proceed with PRK alone, possibly topography-guided, and to monitor her carefully for changes after surgery. Changes would warrant consideration of CXL. I would also discuss the options of LASIK and SMILE with this patient if the other parameters listed earlier are normal, but I would recommend PRK because of the unknown or potentially increased risk of ectasia.

WHAT I DID: CHRISTOPHER E. STARR, MD
Thankfully, all three of the esteemed panelists agree that LVC—PRK in particular—is a reasonable approach in this case because that is the procedure I ultimately performed. I agree that epithelial maps can be useful for assessing a patient’s risk of ectasia, but I did not have access to this technology.
Based on her refractive stability for 8 years, normal corneal thickness profiles, normal posterior floats, 20/15 BCVA OU, and reassuring biomechanical analyses, I thought that the risk of post-LVC ectasia was low enough for us to proceed with PRK. Although increasing the optical and ablation zones can minimize glare and halos in patients with large pupils, this adjustment also increases the total amount of tissue ablated. Because she was not particularly bothered by night vision disturbances preoperatively, I opted to perform wavefront-guided PRK with adjunctive mitomycin C, a standard 6-mm optical zone, and an 8-mm ablation zone.
The patient was read the riot act during the informed consent process, and she is doing well thus far, approximately 8 months postoperatively. UCVA is 20/15 OU. She has exhibited no sign of corneal instability or ectasia. Nor has she reported worsened symptoms of glare, halos, or other low-light visual aberrations.
She is happy, which makes me happy, but of course I will monitor her closely and be ready to perform CXL at the first sign of ectasia.
1. Schallhorn SC. Putting risk/benefit into perspective. Paper presented at: ASCRS Annual Meeting; May 8, 2016; New Orleans.


