A Blue Angle to Remember
Bac T. Nguyen, MD

Trypan blue dye has been safely used to improve visualization in cataract surgery and angle surgery through its staining of the anterior lens capsule and the TM.1-3 My most memorable MIGS case involved an angle that stained so vividly with trypan blue that it fundamentally changed how I teach MIGS to residents.
CASE PRESENTATION
A 70-year-old White woman with POAG and cataracts in both eyes presented with worsening vision that was interfering with her activities of daily living. BCVA was 20/40 OU. IOP was 12 mm Hg OD and 16 mm Hg OS on travoprost 0.04% and timolol maleate 0.5% instilled daily in both eyes. Gonioscopy showed open angles, Shaffer grade IV, with faint pigmentation in both eyes. The patient had 3+ nuclear sclerotic cataracts in both eyes. The cup-to-disc ratio was 0.6 OD and 0.8 OS. VFs were consistent with mild glaucoma in the right eye and moderate glaucoma in the left eye. The patient elected to undergo cataract extraction with IOL implantation along with the placement of a trabecular microbypass stent (Hydrus Microstent, Ivantis) in the left eye.
SURGICAL COURSE
Given the patient’s preoperative gonioscopic finding of faintly pigmented TM, I decided to use trypan blue ophthalmic solution 0.06% (Vision Blue, DORC International) to stain the TM and facilitate implantation of a Hydrus Microstent. After creating two paracenteses for bimanual surgery, I instilled 0.2 mL of preservative-free 1% lidocaine into the anterior chamber. Trypan blue was then injected to fill the anterior chamber and stain the anterior lens capsule and TM for 30 seconds before being irrigated with balanced saline solution. This was followed by successful cataract extraction using phacoemulsification.
After IOL implantation and removal of the OVD from the capsular bag complex, 0.1 mL of carbachol intraocular solution 0.01% (Miostat, Alcon) was instilled in the anterior chamber to induce miosis. This was followed by an injection of OVD to inflate the anterior chamber and nasal angle. The operating microscope was tilted 45º toward the surgeon, and the patient’s head was rotated 30º away from the surgeon to aid in visualization of the nasal angle for the MIGS procedure. A left-handed Swan Jacob gonioprism was placed on the cornea with an OVD coupling agent. The TM was readily identifiable by its bright blue color from the trypan blue staining.
I introduced the Hydrus Microstent injector into the anterior chamber through the temporal corneal wound. The injector cannula tip was engaged into Schlemm canal, and the Hydrus Microstent was deployed into the canal by scrolling the injector wheel forward. The injector lock was disengaged from the implant, and the injector was removed from the anterior chamber. I used a Sinskey hook to adjust the positioning of the device and then removed the remaining OVD. At 6 months postoperative, the patient’s UCVA was 20/25, and IOP was 12 mm Hg on travoprost 0.04% and timolol maleate 0.5% daily.
CONCLUSION
Although it has long been documented that trypan blue aids in enhances visualization for cataract surgery, recent advances in angle-based glaucoma surgery in adults have shown new uses for this vital dye, including staining of the TM and outlining of the collector channels.1-3 I personally had not seen a TM stain so brightly and vividly until this particular case. Most impressively, the staining persisted until the end of the procedure, despite having been applied before cataract extraction. The views seen in my video demonstration (see Watch It Now) were captured after cataract extraction and IOL implantation.
Dr. Nguyen instills trypan blue for angle visualization.
1. Melles GR, Waard PWD, Pameyer JH, et al. Trypan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg. 1999;25:7-9.
2. Grover DS, Fellman RL. Confirming and establishing patency of glaucoma drainage devices using trypan blue. J Glaucoma. 2013;22:1-2.
3. Laroche D, Nortey A, Ng C. A novel use of trypan blue during canalicular glaucoma surgery to identify aqueous outflow to episcleral and intrascleral veins. J Glaucoma. 2018;27:158-161.
Mixing MIGS During COVID-19
I. Paul Singh, MD

Glaucoma specialists today are fortunate to practice in an age with a wide variety of available treatment options. During the COVID-19 pandemic especially, I have found having access to a range of MIGS devices to be extremely valuable for addressing the individual needs and concerns of each patient who presents for glaucoma care.
This time has posed a unique set of challenges to physicians, patients, and practices alike. Over the past few months, the specific concerns on my mind have been the following:
- Identifying which patients are at risk of permanent vision loss;
- Minimizing postoperative events;
- Minimizing postoperative visits;
- Minimizing postoperative drops and compliance issues; and
- Navigating care with a leaner staff.
In light of these additional challenges, the importance of maximizing both the efficacy and safety of glaucoma treatments has been heightened. One way to achieve this goal for surgical glaucoma patients is by combining MIGS approaches with different mechanisms of action.
With conventional outflow MIGS, we do not know where the resistance to outflow is preoperatively. Is it blockage in the TM or a collapse of Schlemm canal? Are there herniations into the collector system? We have a range of individual approaches—including placement of outflow stents, dilation of the outflow system, stripping or removal of the TM—that work at different levels of the outflow system. Combining approaches potentially allows us to maximize outflow while maintaining the safety of conventional angle-based MIGS procedures.
CASE NO. 1
A 78-year-old patient presented with BCVA of 20/60 OD and 20/40 OS. The patient had ocular surface disease and a significant cataract in the right eye. Maximum IOP was 39 mm Hg OD and 33 mm Hg OS. IOP on four medications was 32 mm Hg OD and 22 mm Hg OS. Gonioscopy revealed an open angle and +2 pigmentation of the TM. The patient had a history of a failed Ex-Press Glaucoma Filtration Device (Alcon) in the right eye, rendering the conjunctiva unhealthy, as well as a history of failed selective laser trabeculoplasty in both eyes, indicating likely resistance behind the TM, either in Schlemm canal or distal channels. Corneal hysteresis was low, at around 7 OD and 8 OS (compared with a normal value of 10). This indicated that the shock-absorbing ability of the patient’s cornea might be poor, placing him at greater risk of glaucomatous progression.
Pachymetry was fairly normal, with a central corneal thickness of 523 μm OD and 534 μm OS. On OCT, however, the ganglion cell layer was almost negligible, the ganglion cell complex was wiped out, and the retinal nerve fiber layer in both eyes was extremely thin (Figure 1). The optic nerve in the right eye had a notch in the inferior rim. VF testing revealed paracentral loss with a superior defect in the right eye that was consistent with the retinal nerve fiber layer loss and disc notch. Moderate disease was also indicated in the left eye by VF testing.
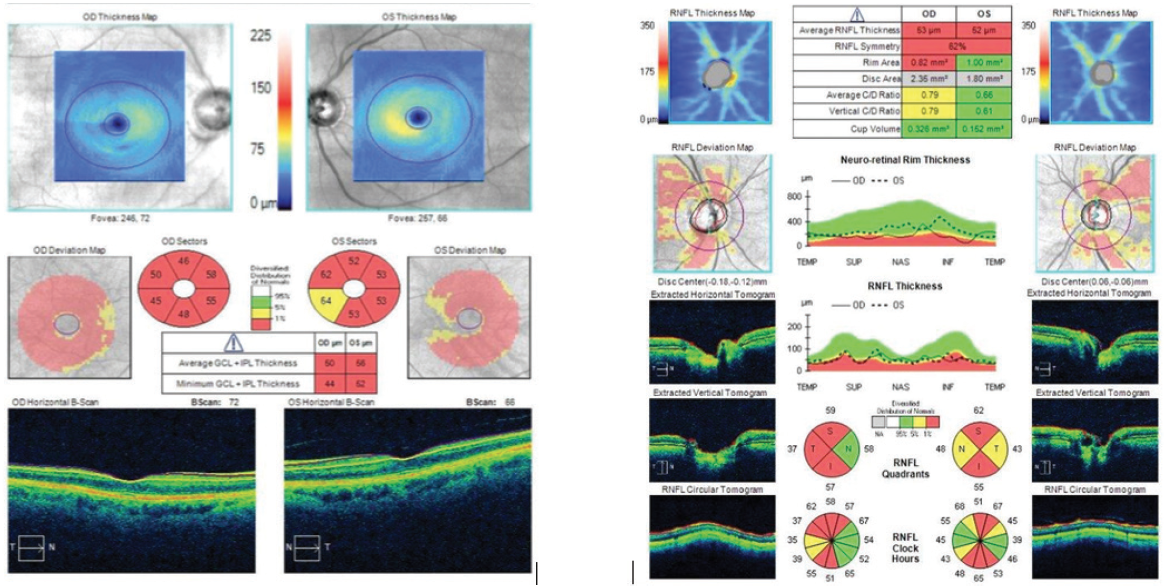
Figure 1. The patient’s ganglion cell layer was almost negligible, the ganglion cell complex was wiped out, and the retinal nerve fiber layer in both eyes was extremely thin.
Given this diagnostic picture and the patient’s concerns about coming into the office, I decided to use a combined approach, including 360º viscodilation with the Omni Surgical System (Sight Sciences), 180º goniotomy, implantation of a Hydrus Microstent, and cataract extraction (see Watch It Now). With the Omni device, I engaged the TM at about a 45º angle and advanced the nylon thread 180º in one direction. As the thread was retracted, OVD was released to dilate the canal and distal channels. I then performed the same technique to address the other 180º, but after viscodilation, I rethreaded the canal and then performed a 180º goniotomy. Next, addressing the part of the angle that was not cut, I placed a Hydrus stent, which not only bypasses the TM but also scaffolds Schlemm canal. Performing viscodilation first enables the implant to slip in beautifully.
Dr. Singh performs combination surgery with Omni and Hydrus devices.
Dr. Singh performs combination surgery with Omni and iStent inject devices.
In this case, I was able to maximize distal outflow via viscodilation, cut the TM about 180º, and, in the other 180º, place a scaffolding device to maximize outflow through the conventional pathway. On postoperative day 1, IOP was 18 mm Hg. At postoperative month 1, IOP was 14 mm Hg, and UCVA was 20/30 on brimonidine-timolol only. For this 78-year-old patient with an IOP in the 30s on four medications, by mixing MIGS procedures, I was able to maximize efficacy, decrease IOP and the number of glaucoma medications, and minimize the number of postoperative visits required compared with traditional glaucoma surgery.
CASE NO. 2
I also recently performed 360º viscodilation with concomitant placement of iStent inject (Glaukos) in a patient with open-angle glaucoma who was undergoing cataract surgery. This 68-year-old woman had mild POAG on brimonidine, timolol, and bimatoprost. She had preoperative IOPs in the low 20s with some fluctuation. She had documented progressive VF loss and a worsening cataract. Central corneal thickness was near 550 μm OU, and corneal hysteresis was around 9 OU. The patient admitted to being noncompliant with her medications.
As shown in my second video, once the patient’s cataract was removed, I obtained a beautiful view of the angle with a gonioprism, scored the TM with the Omni loader, and then aimed at about a 45º angle toward the posterior wall. I advanced the wheel to release the nylon thread into Schlemm canal. Once the wheel stopped, I reversed the wheel to retract the thread and release OVD. Going back the other way, I also engaged at about a 45º angle, advanced the wheel to release the nylon thread into Schlemm canal for the other 180º, and then retracted the wheel and nylon thread while OVD was released. This maneuver helped to dilate Schlemm canal and the distal channels.
Next, I placed the first iStent inject as perpendicular to the TM as possible while dimpling down to ensure optimal placement. About 2 clock hours away, I placed the second iStent inject. One must be careful not to dimple too much because the canal has been dilated and there is a risk of overimplantation. During irrigation and aspiration, a nice fluid wave appeared near the site of the implants, verifying that outflow had increased. After removing the OVD, I hydrated the wounds and ensured that the pressure was high enough (in the mid- to upper teens) to prevent blood reflux.
The patient’s vision improved quickly, from 20/30 on postoperative day 1 to 20/20 at postoperative week 1. IOP was 15 mm Hg on postoperative day 1, 17 mm Hg at postoperative week 1, and 16 mm Hg at postoperative month 1 off all medication. No additional or unscheduled visits were required.
A patient administering more than two or three medications, even with mild disease, is one for whom it may make sense to combine mechanisms to maximize outflow yet save the TM for a future procedure such as selective laser trabeculoplasty or a cutting/stripping procedure as needed.
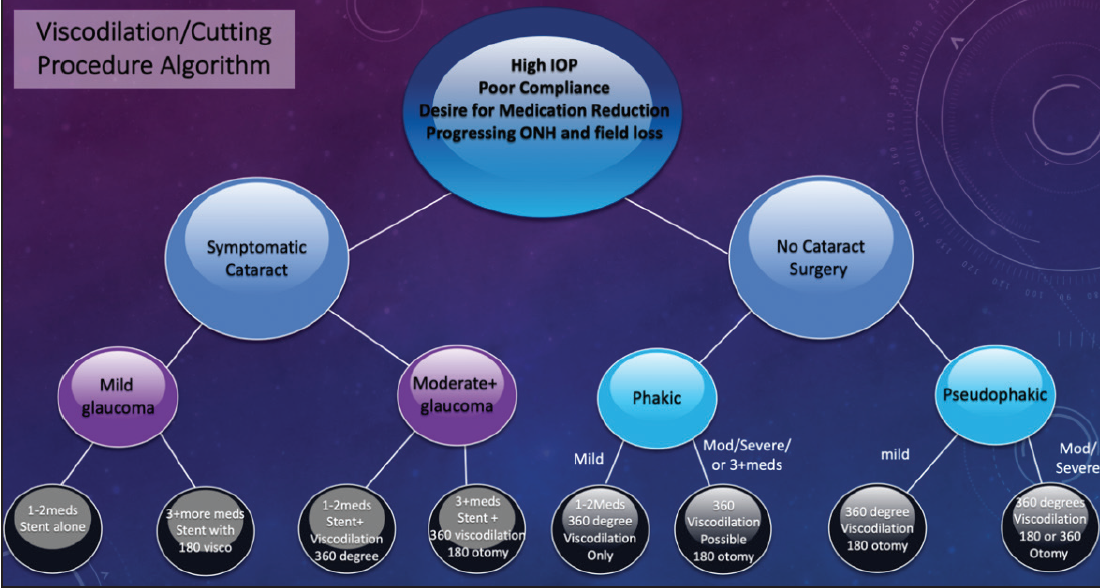
Figure 5. Dr. Singh’s treatment algorithm for mixing MIGS procedures.
Figures 1 and 2 courtesy of I. Paul Singh, MD
CONCLUSION
Mixing MIGS procedures can be extremely valuable in certain cases. Figure 2 shows my go-to algorithm for working through this decision-making process in order to identify the surgical approach that achieves optimal safety and efficacy for each patient.
*The views expressed in this article are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US Government.



