

Since their introduction, corneal laser procedures have been the workhorse of refractive surgery. In the late 1990s, concerns over pain, recovery time, and subepithelial haze associated with PRK fueled a shift toward LASIK, which quickly moved into the forefront with faster recovery times, less associated pain, and improved outcomes.
LASIK initially introduced a number of variables, as mechanical microkeratomes produced flaps with relatively large standard deviations in thickness and diameter. The appearance of post-LASIK ectasia introduced concerns regarding appropriate guidelines for safe treatment.
Fortunately, because LASIK has remained the main refractive procedure since its introduction, a large amount of data has been generated and analyzed. Additionally, technology to image and analyze the cornea is continually being upgraded, and improvements in microkeratomes and the development of femtosecond lasers for flap creation have reduced surgical variability.
Despite these developments, controversy remains regarding the limits of LASIK and the most appropriate technologies to determine corneal safety. In this article, we outline the guidelines we use at our practice in Hawaii to identify appropriate candidates for LASIK.
PATIENT WORKUP
Every patient we see for a refractive surgery screening has a thorough workup consisting of a discussion about hobbies, lifestyle, and visual goals. We obtain patients’ medical and eye history and perform diagnostic testing and an undilated eye exam. We inquire about refractive stability, especially for younger patients.
We consider it essential to review anterior and posterior corneal topography. We examine the Belin/Ambrósio Enhanced Ectasia Display on the Pentacam (Oculus Optikgeräte). We perform corneal aberrometry and LipiScan dynamic meibomian imaging (Johnson & Johnson Vision) on every patient, and we use the HD Analyzer (Visiometrics) in patients older than 45 years. For suspicious corneas or corneal scars, we obtain epithelial thickness mapping or anterior segment OCT.
Although each element in this array of diagnostic imaging devices may not be essential in every patient, we find that each one provides valuable information for certain patients and in certain diagnostic dilemmas.
RANGE OF TREATMENT
Depending on the platform, in the United States, current approvals for LASIK allow treatment of myopia up to -12.00 D, astigmatism up to 6.00 D, and hyperopia up to 6.00 D. Our experience is based primarily on wavefront-optimized treatments. Depending on the applied treatment pattern (ie, the laser platform), treatment at the extremes of those ranges may induce more aberrations, more dry eye disease (DED), and more dysphotopsias.
In our opinion, a comfortable range of myopia correction with LASIK is up to -8.00 D. Above that, we tend to favor lens-based procedures—a phakic IOL such as the EVO Visian ICL (STAAR Surgical) in young patients or refractive lens exchange (RLE) for patients in the presbyopia age range. Although we have treated myopia as great as -12.00 D with LASIK, these treatments are less predictable and have a higher incidence of side effects.
Many surgeons advocate performing PRK in patients with thin corneas outside the range for LASIK, but this should be done cautiously, especially in sunny areas (like Hawaii) with a higher risk of postoperative haze. Therefore, we tend to favor the ICL over PRK for high corrections in eyes with thin or abnormal corneas. Several studies, including a contralateral eye study conducted years ago, showed that, with myopia greater than -6.00 or -8.00 D, patients preferred ICL over LASIK.1,2 ICL implantation was also found to be more stable than LVC for patients with high and extreme myopia, which is consistent with the results of previous studies.3,4
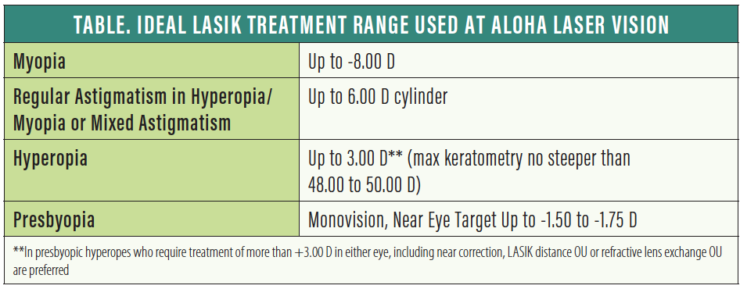
We are comfortable treating regular astigmatism up to the approved 6.00 D in both hyperopia and myopia. Certainly, the higher the cylinder, the more challenging it is to obtain the desired outcome. Higher cylinder ablations result in a reduced effective optical zone on some laser platforms, increasing the risks of induced glare and aberrations.
Treatment of hyperopia depends significantly on age for a number of reasons. First, in contrast to myopes, hyperopes undergo progression, especially in the postpresbyopic years. Second, after hyperopic LASIK, patients can end up with a hyperprolate cornea that can be optically unsuitable for multifocality. For these reasons, we prefer RLE for hyperopic patients in the presbyopic age range.
For younger hyperopes, we are comfortable treating hyperopia up to 3.00 D, and less comfortable from 3.00 to 4.00 D. We have treated up to 6.00 D, but rarely. Some of the most successful patients we have had were highly hyperopic and with mixed cylinders, which we treat up to the full approved range. Although we do not have a minimum or flattest keratometry (K) value with myopic LASIK, we prefer to keep max K’s no steeper than 48.00 to 50.00 D after hyperopic LASIK.
PRESBYOPIA
Numerous ablation patterns have been proposed for the treatment of presbyopia, but none have been any more successful than monovision or blended vision. (Editor’s note: For more on laser blended vision, see Choosing a Method of Presbyopic Correction in Patients Without Visually Significan Cataracts.) We tend to favor using a myopic target of up to -1.50 to -1.75 D, usually in the nondominant eye, based on preoperative demonstration and refinement. We find that monovision is better tolerated and longer lasting in myopes than in hyperopes.
In hyperopic presbyopes, we favor RLE with trifocal IOLs as a definitive treatment of both refractive error and presbyopia. The Light Adjustable Lens (RxSight) and other lens combinations, including monovision corrections, can also be successfully used with RLE.
Although we sometimes correct only one eye in a monovision patient, it is important that the distance eye have excellent UCVA. Poor distance UCVA is a common reason patients are dissatisfied with monovision. This requires treating any hyperopia in the distance vision eye, even if the uncorrected vision is good, because any accommodation of the distance eye also affects the near vision eye.
SCREENING AND DETERMINING CANDIDACY
Determining corneal health for LASIK remains an area of great challenge and controversy. Because infections and severe inflammation after LASIK are exceedingly rare, the most feared complication today is corneal ectasia.
Numerous technologies and analytical programs have been developed to identify corneas at risk for ectasia. Nonetheless, careful evaluation of corneal tomography, looking at both the anterior and posterior corneal surfaces, is still the most accepted screening method for signs of ectasia. There are some proponents of epithelial mapping5 and ocular hysteresis measurement,6 and there are some data to support these measures, but neither is in widespread use at this time.
Opinions and practice patterns vary, but it is widely believed that maintaining a residual stromal thickness (RST) of more than 250 µm and percentage of tissue altered (PTA) of less than 40% can reduce the risk of keratoconus.7 Although there is no evidence that the 250-µm limit suggested by the US FDA is not adequate, many surgeons try to maintain 300 µm as the minimum residual bed thickness. Many surgeons also limit LASIK to patients with preoperative corneal thickness greater than 500 µm, but studies have not supported increased risk based on this alone. One study found LASIK in corneas below 500 µm to be safe.8
It is important to remember that these cutoff values assume that the cornea in question is normal, without preexisting keratoconus, as most instances of post-LASIK ectasia are now thought to be due to progression of preexisting keratoconus. Because the two main risk factors for ectasia are preexisting keratoconus and removal of excess tissue, it is worthwhile to consider RST and PTA as additional data points in assessing a patient’s candidacy for LASIK. These cutoff values, however, should not be taken as absolutes to be used in isolation.
Based on a 2014 analysis, Santhiago and colleagues stated that, of the risk factors they considered, a PTA of 40% or more carried the greatest risk of post-LASIK ectasia (odds ratio, 223).9 In such cases, those authors said, they perform PRK. However, other studies have failed to validate these results.
Relying on Randleman’s ectasia score10 or using PTA or RST cutoffs alone would have disqualified many patients who have had LASIK successfully in the past, at our practice and those of other well-respected refractive surgeons. For instance, Reinstein and colleagues performed a retrospective analysis of more than 15,000 patients treated since 2002. If they had applied 40% PTA as a cutoff, they determined, 26.5% of their total database would have been denied LASIK surgery. In that database, only seven cases of ectasia had developed.11
Although we try to respect the PTA and RST safety margins, these numbers are guides and data points to be used in combination with other factors. In our opinion, careful assessment of the cornea is the most important risk factor for avoiding ectasia. For patients with mildly irregular topographies, we are comfortable performing PRK. For true keratoconus, we recommend CXL followed by ICL implantation or PRK. Studies have shown that PRK may be safe in keratoconus.12
FURTHER SCREENINGS
All potential LASIK patients should be screened for DED and meibomian gland dysfunction (MGD). Patients with DED secondary to systemic disease should be assessed carefully and are more concerning. The majority of DED in potential LASIK patients, however, is MGD or limbal stem cell deficiency caused by contact lens wear.
Demonstration of changes in the meibomian glands via imaging greatly assists in counseling patients regarding the risk of postoperative symptoms. We often explain to patients with postoperative symptoms of dryness that they are recovering from both the LASIK procedure and their contact lens wear.
OTHER ISSUES
Breastfeeding. Breastfeeding and pregnancy can cause refractive instability and worsened DED symptoms. We do not perform LASIK in pregnant women but will do so during breastfeeding if the patient did not have refractive changes during pregnancy. Precautions such as encouraging the patient to pump and dump their breastmilk or to avoid oral anxiolytics may be needed in these cases.
Autoimmune disease. Both the AAO and the US FDA cite autoimmune and immunodeficiency conditions as contraindications for LASIK. However, LASIK may be reasonably safe in patients with well-controlled or inactive autoimmune disease or HIV who are otherwise good candidates. This has been our experience and has been reported by other refractive surgeons.13,14 Of course, proper informed consent must be obtained.
Diabetes. Diabetes can cause refractive instability, but, in our experience, wound healing has not been an issue in patients with well-controlled diabetes. For those with either autoimmune disease or diabetes, we prefer LASIK over PRK.
Glaucoma. Patients should be informed that any corneal refractive procedure could make measuring their IOP more challenging. However, given that diagnosing and following glaucoma requires other diagnostic testing, IOP is not the only data point involved. Many refractive surgeons will perform LASIK or PRK in glaucoma patients after a thorough discussion of the risks. Some argue that PRK is safer because it avoids the brief IOP rise during femtosecond laser flap creation and eliminates the risk of interface fluid syndrome. Practically speaking, most patients with glaucoma are older and can have their vision corrected with lens-based procedures.
Isotretinoin. Patients’ use of isotretinoin for treatment of acne can significantly worsen postoperative DED. We prefer to wait at least 1 year after discontinuation of this medication before considering LASIK—if at all.
Occupational issues. Determining the patient’s occupation and vocational activities is an important part of the preoperative assessment. Patients with a higher risk of direct trauma to the cornea are of the greatest concern. Specifically, this includes most martial artists and fighters. Other sports of concern include basketball and those in which finger-induced eye injuries are common.
At our practice in Hawaii, we ask our patients to remain out of the water for only 1 week. Despite the extremes of surfing, we have not seen any flap injuries or infections.
Asking about occupation and vocation also provides insights into patients’ activities and may influence your choice of refractive target and furnish clues to potential postoperative issues such as prolonged screen use or other environmental issues.
CONCLUSION
LASIK is the most commonly performed vision correction procedure in the United States. Given that most of our refractive surgical screening patients come in seeking LASIK, and that almost all have known a happy LASIK patient, there is a tendency for us to try to satisfy patients by qualifying them as LASIK candidates. That being said, however, there are times when other vision correction procedures are better.
In our practice, we have found that, when we explain the rationale for proposing an alternative procedure—for example PRK or ICL in a patient with an abnormal cornea—patients are generally accepting and even appreciative that we prioritize their safety over convenience.
There are many fantastic procedures for correcting vision. Our goal as refractive surgeons is to make patients happy by correcting their vision safely. Choosing the right procedure for the right patient is arguably more challenging, and more nuanced, than learning to perform the surgeries themselves. We hope the guidelines outlined here will help you to make the right recommendations for your patients seeking vision correction.
Choosing a Method of Presbyopic Correction in Patients Without Visually Significant Cataracts
By Dan Z. Reinstein, MD, MA(Cantab), FRCSC, DABO, FRCOphth, FEBO
Modern laser platforms can be used to treat nearly every patient who walks through the door, including presbyopes.1-3 In the presence of a visually significant cataract, intraocular surgery is the only refractive surgical option, but I believe that corneal laser refractive surgery is safer for a patient with a clear lens.
Refractive lens exchange (RLE) is becoming more popular as a result of improvements in phacoemulsification, fluidics, and capsulotomy technology, but safety remains my top priority. If it can be shown, for example by measuring the optical scatter index on the HD Analyzer (Visiometrics), that a patient’s visual quality remains high, then corneal laser surgery is the better, safer option.
MYTHS AND CONCERNS
There are several myths and inaccuracies in the information provided to patients regarding RLE.
Myth No. 1: RLE provides permanent vision correction. Actually, the cornea is reshaped continuously after 40 years of age, and this change in cylinder will affect the prescription in about one-third of all patients.4,5 Therefore, although the lens replacement itself is permanent, the procedure should not be sold as a permanent correction of vision.
Myth No. 2: Everyone will develop a cataract, so replacing a clear natural lens will prevent cataracts. In the United Kingdom, only 30% of the predominantly Anglo-Saxon population will develop a visually significant cataract requiring surgery.6 On the other hand, with laser refractive surgery, enhancements are possible for most patients on an ongoing basis to keep vision optimized and patients out of spectacles until the day that a cataract develops.
Another issue in the choice of refractive surgical approach is the risk of visually significant complications with phaco surgery compared to that with laser corneal refractive surgery. It is intuitive to most lay people that going inside the eye to replace a lens that is still transmitting light (ie, no cataract) is more invasive than performing an extraocular laser procedure on the surface of the eye; nonetheless, this point is often overlooked or minimized by the surgeon. Catastrophic risks such as major bleeding or infection are rare.7,8 However, RLE patients are generally younger than patients undergoing cataract surgery, so the former must understand that they have a higher risk for retinal detachment and cystoid macular edema as a result of an attached vitreous. Two large national studies concluded that the risk for retinal detachment after lens surgery is highest for patients younger than 60 years of age, and the risk increases by a factor of 20 for a 50-year-old compared to an 80-year-old.9,10
Finally, one must consider evolving IOL technology. Although current presbyopia-correcting IOL technology is very good, there are still limitations, including decreased contrast sensitivity and an increase in glare and halo at night. For patients with already decreased contrast and night vision disturbances due to cataract, these are reasonable side effects. But there will probably be much better IOL options available in the next 10 to 20 years in the form of monofocal IOLs that accommodate without decreasing contrast or increasing night vision disturbances.
SAFETY AND GOOD RESULTS
The outcomes that we can achieve with laser blended vision for presbyopia are equal to and generally better than the vast majority of reported RLE outcomes for myopia, hyperopia, and emmetropia.11,12 In our clinic 95% of myopic (up to -8.50 D), 80% of hyperopic (up to +5.00 D), and 98% of emmetropic patients with presbyopia end up with 20/20 at distance and can read newspaper print (J5) after Presbyond LASIK (Carl Zeiss Meditec). In those same groups, 95%, 68%, and 96%, respectively, achieve 20/20 distance and J1 reading.13-15
And these results are achieved without patients having to deal with glare and halos at night or worry about the other risks associated with RLE.
1. Reinstein DZ, Carp GI, Lewis TA, Archer TJ, Gobbe M. Outcomes for myopic LASIK with the MEL 90 excimer laser. J Refract Surg. 2015;31:316-321.
2. Pradhan KR, Reinstein DZ, Carp GI, Archer TJ, Gobbe M, Dhungana P. Quality control outcomes analysis of small-incision lenticule extraction for myopia by a novice surgeon at the first refractive surgery unit in Nepal during the first 2 years of operation. J Cataract Refract Surg. 2016;42:267-274.
3. Reinstein DZ, Carp GI, Archer TJ, et al. Long-term visual and refractive outcomes after LASIK for high myopia and astigmatism from -8.00 to -14.25 D. J Refract Surg. 2016;32:290-297.
4. Ueno Y, Hiraoka T, Beheregaray S, Miyazaki M, Ito M, Oshika T. Age-related changes in anterior, posterior, and total corneal astigmatism. J Refract Surg. 2014;30:192-197.
5. Hashemi H, Asgari S, Emamian MH, Mehravaran S, Fotouhi A. Age-related changes in corneal curvature and shape: The Shahroud Eye Cohort Study. Cornea. 2015;34:1456-1458.
6. Minassian DC, Reidy A, Desai P, Farrow S, Vafidis G, Minassian A. The deficit in cataract surgery in England and Wales and the escalating problem of visual impairment: epidemiological modelling of the population dynamics of cataract. Br J Ophthalmol. 2000;84:4-8.
7. Ling R, Cole M, James C, Kamalarajah S, Foot B, Shaw S. Suprachoroidal haemorrhage complicating cataract surgery in the UK: epidemiology, clinical features, management, and outcomes. Br J Ophthalmol. 2004;88:478-480.
8. Day AC, Donachie PH, Sparrow JM, Johnston RL. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 1, visual outcomes and complications. Eye (Lond). 2015;29:552-560.
9. Daien V, Korobelnik JF, Delcourt C, et al. French Medical-Administrative Database for Epidemiology and Safety in Ophthalmology (EPISAFE): The EPISAFE Collaboration Program in Cataract Surgery. Ophthalmic Res. 2017;58:67-73.
10. Clark A, Morlet N, Ng JQ, Preen DB, Semmens JB. Risk for retinal detachment after phacoemulsification: a whole-population study of cataract surgery outcomes. Arch Ophthalmol. 2012;130:882-888.
11. Power B, Murphy R, Leccisotti A, Moore T, Power W, O’Brien P. Maximising refractive outcomes with an extended depth of focus IOL. Open Ophthalmol J. 2018;12:273-280.
12. Chang JS, Ng JC, Chan VK, Law AK. Visual outcomes and patient satisfaction after refractive lens exchange with a single-piece diffractive multifocal intraocular lens. J Ophthalmol. 2014;2014:458296.
13. Reinstein DZ, Archer TJ, Gobbe M. LASIK for myopic astigmatism and presbyopia using non-linear aspheric micro-monovision with the Carl Zeiss Meditec MEL 80 Platform. J Refract Surg. 2011;27:23-37.
14. Reinstein DZ, Carp GI, Archer TJ, Gobbe M. LASIK for the correction of presbyopia in emmetropic patients using aspheric ablation profiles and a micro-monovision protocol with the Carl Zeiss Meditec MEL80 and VisuMax. J Refract Surg. 2012;28:531-541.
15. Reinstein DZ, Couch DG, Archer TJ. LASIK for hyperopic astigmatism and presbyopia using micro-monovision with the Carl Zeiss Meditec MEL80. J Refract Surg. 2009;25:37-58.
1. Parkhurst GD, Psolka M, Kezirian GM. Phakic intraocular lens implantation in United States military warfighters: A retrospective analysis of early clinical outcomes of the Visian ICL. J Refract Surg. 2011;27(7):473-481.
2. Chen X, Guo L, Han T, Wu L, Wang X, Zhou X. Contralateral eye comparison of the long‐term visual quality and stability between implantable collamer lens and laser refractive surgery for myopia. Acta Ophthalmol. 2019;97:e471-e478.
3. Igarashi A, Kamiya K, Shimizu K, Komatsu M. Visual performance after implantable collamer lens implantation and wavefront‐guided laser in situ keratomileusis for high myopia. Am J Ophthalmol. 2009;148:164-170.e1.
4. Sanders D, Vukich JA. Comparison of implantable collamer lens (ICL) and laser-assisted in situ keratomileusis (LASIK) for low myopia. Cornea. 2006;25:1139-1146.
5. Schallhorn JM, Tang M, Li Y, Louie DJ, Chamberlain W, Huang D. Distinguishing between contact lens warpage and ectasia: Usefulness of optical coherence tomography epithelial thickness mapping. J Cataract Refract Surg. 2017;43(1):60-66.
6. Moshirfar M, Edmonds JN, Behunin NL, Christiansen SM. Corneal biomechanics in iatrogenic ectasia and keratoconus: A review of the literature. Oman J Ophthalmol. 2013;6(1):12-17.
7. Giri P, Azar DT. Risk profiles of ectasia after keratorefractive surgery. Curr Opin Ophthalmol. 2017;28(4):337-342.
8. Kymionis GD, Bouzoukis D, Diakonis V, et al. Long-term results of thin corneas after refractive laser surgery. Am J Ophthalmol. 2007;144(2):181-185.
9. Santhiago MR, Smadja D, Gomes BF, et al. Association between the percent tissue altered and post-laser in situ keratomileusis ectasia in eyes with normal preoperative topography. Am J Ophthalmol. 2014;158(1):87-95.e1.
10. Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37-50.
11. Hillman L. Presentation spotlight: Considering percent tissue altered as a risk factor for post-LASIK ectasia. Eyeworld. May 2018.
12. Guedj M, Saad A, Audureau E, Gatinel D. Photorefractive keratectomy in patients with suspected keratoconus: five-year follow-up. J Cataract Refract Surg. 2013;39(1):66-73.
13. Simpson RG, Moshirfar M, Edmonds JN, Christiansen SM, Behunin N. Laser in situ keratomileusis in patients with collagen vascular disease: a review of the literature. Clin Ophthalmol. 2012;6:1827-1837.
14. Aref AA, Scott IU, Zerfoss EL, Kunselman MA. Refractive surgical practices in persons with human immunodeficiency virus positivity or acquired immune deficiency syndrome. J Cataract Refract Surg. 2010;36:153-160.



