Monovision, as a strategy for addressing presbyopia, has been used in contact lenses since the 1960s in Ireland. The Wellington Eye Clinic started implementing monovision in laser vision correction shortly after the introduction of excimer lasers, and, by 1998, monovision was in common use in our clinic.
Over time, we developed a diagnostic workup routine that gave us a very good indication as to whether a patient was suitable for monovision or not (http://bit.ly/Cummings0619). With the patient binocularly corrected for distance (reference = 100%), we added near vision correction in the nondominant eye with the auto-phoropter. We used preselected adds (0.00 and -1.00 D for mini-monovision and 0.00 and -1.75 D for full monovision) and asked the patient to score the monovision selection for distance viewing. If the score was above 80%, the odds were high that monovision would work. If the score was below 80%, the odds decreased significantly.
One thing was certain, though: When the target for the reading eye was -1.75 D, that eye’s uncorrected distance visual acuity (UDVA) was typically in the range of 6/48 Snellen, or 20/160 (0.9 logMAR). Despite this poor UDVA in the reading eye, for patients with a good aptitude for monovision, their binocular UDVA was satisfactory, and this was then a small sacrifice for the bonus of greatly increased reading or near vision.
CORNEAL INLAYS
In the past decade, several corneal inlays have aimed to provide a modified form of monovision presbyopia correction. These include the Kamra (CorneaGen), the Raindrop (ReVision Optics; no longer available), and the Presbia Flexivue Microlens (Presbia; withdrawn from the market). Of the three, I have had experience with the Kamra and the Raindrop. Both provided reading vision with less loss of UDVA in the reading eye than is possible with monovision. Over some months of neural adaptation, most patients with these inlays reached a point when they felt as though there was no loss of UDVA binocularly but there was a significant improvement in near vision.
When these inlays worked, they gave very satisfactory results. The Raindrop was well tolerated optically but not so well tolerated by the cornea. The Kamra was better tolerated by the cornea, but, in my view, too many patients simply were not happy with their vision.
Those who were happy with these implants were truly happy with their vision, but, despite being meticulous in patient selection, I could not refine the selection process to the point where the number of patients who were unhappy was low enough to warrant the device’s use. Although I never implanted the Presbia inlay, I have heard similar comments about that implant from colleagues. In the meantime, the Raindrop has been discontinued, and the Presbia has been withdrawn from the market. The Kamra is still available, and surgeons continue to use it and to report satisfactory outcomes.
A NEW OPTION
In my experience, patient satisfaction was higher with the Raindrop than with the Kamra. Therefore, when an allograft product that worked on the same principles as the Raindrop became available, I was eager to participate in a European clinical trial of the device sponsored by the manufacturer.
The allogenic lenticules (TransForm Corneal Allograft, Allotex) are obtained from an eye bank and processed by Allotex into 2.50 D add lenses with a diameter of 2.65 mm and a central thickness of around 29 µm. The lenticules for the ongoing study in which I am participating are provided by Allotex.
The optical effect of the TransForm inlay produces a controlled induction of negative spherical aberration in the implanted eye, allowing light from distance to enter the eye around the inlay through a large pupil. When the pupil constricts with convergence and attempted accommodation, only the part of the corneal optics that includes the lenticule is involved, and there is higher power for reading.
In my experience, this is equivalent to what one would find with a -2.00 D target in a monovision eye. The difference, however, is that the UDVA in the reading eye is in the range of 6/12 Snellen (0.5 or 0.3 logMAR) or better rather than the 6/30 to 6/60 Snellen range (0.7 to 1.0 logMAR).
CAIRS for Keratoconus and Pearl for Presbyopia
New treatments using allogenic donor tissue are on the horizon.
By Soosan Jacob, MS, FRCS, DNB

I have developed two novel allogenic donor cornea therapies, one for the treatment of keratoconus and one for the treatment of presbyopia.
CAIRS. Corneal allogenic intrastromal ring segment (CAIRS) implantation can be performed in isolation or in combination with CXL. For this procedure, intrastromal corneal ring segments (ICRSs) are fashioned from human donor corneal tissue and implanted in a manner similar to that for standard synthetic ICRSs. Depending on whether the keratoconus is progressive or not, CAIRS can be combined with CXL to strengthen the cornea. Using donor corneal tissue instead of synthetic ICRSs avoids the possible complications associated with implanting synthetic material in the cornea, including implant extrusion, intrusion or migration; neovascularization; corneal melt; corneal necrosis; and infection.
Each allogenic ICRS is obtained from a donor corneoscleral rim that has been de–endothelialized and de–epithelialized and cut to shape with a double-bladed trephine (patent pending). Allogenic ICRSs are more flexible than synthetic ones. Because they are more biocompatible than ICRSs, they are less likely to be extruded or migrated and they can be inserted at 50% corneal depth rather than at 75% to 80%, as with synthetic implants. The shallower insertion plane can enhance the ability of the ICRS to reshape the cornea, and it can be used in a wider range of eyes with more severe thinning and/or steepening.
In the CAIRS procedure, a femtosecond laser–dissected channel is created with two entry incisions 180º apart. The allogenic tissue is pushed in gently from one side of the channel arc with a blunt rod and then pulled in from the other side with a reverse Sinskey hook. The same is repeated with the second segment for the other half arc of the channel. The ends are trimmed and positioned within the channels. After the segments are in place, epithelium-off accelerated CXL or contact lens–assisted CXL is performed, depending on the minimum corneal thickness. (For a primer on the technique, see http://bit.ly/Jacob0619 and http://bit.ly/Jacob0619b).
I have performed CAIRS in a large series of patients. In my experience, patients achieve good refractive and topographic outcomes, including regularization of the cornea and centralization of the cone, decreased aberrations, and decreased regular and irregular astigmatism (Figure 1). I have used the technique for all stages of keratoconus, from mild to advanced, with very good results.

Figure 1. Preoperative (left), postoperative (middle), and topographical difference maps (right) after CAIRS implantation.
PEARL. Another procedure I have developed is presbyopic allogenic refractive lenticule (PEARL) implantation. PEARL is designed to treat presbyopia by using allogenic donor tissue. For this purpose, we used a lenticule of suitable thickness obtained from a small-incision lenticule extraction procedure performed on a young myopic donor planned for refractive surgery, as it was easy to obtain in the absence of other precisely shaped and sized allogenic tissue.
Once implanted, the PEARL inlay alters the cornea’s central radius of curvature and results in a hyperprolate central corneal contour. Made of allogenic material, the biocompatible inlay allows oxygen and nutrients to pass freely through the cornea, unlike some synthetic corneal inlays. This ensures a stable cornea, decreases the risks of corneal necrosis and melt that have been seen with synthetic inlays, and avoids the inflammation related to insertion of a synthetic material into the cornea.

Figure 2. The PEARL lenticule (arrow) is inserted under a cap in the patient’s nondominant eye.
To perform PEARL, the donor lenticule is marked at its center and cut with a trephine to a 1-mm diameter. It is then implanted in the cornea underneath a 120-µm femtosecond laser cap, centered on a coaxially sighted light reflex (Figure 2). The lenticule is implanted in the nondominant eye to provide improved near and intermediate vision. (For a primer on the technique, see http://bit.ly/Jacob0619c). In my experience, patients have good binocular uncorrected distance and near visual acuity, but they do need to be cautioned to expect some decrease in uncorrected distance visual acuity in the treated eye.
METHOD
To prepare the lenticule, the package supplied by Allotex is opened, and the lenticule is bathed in balanced saline solution to wash away the albumin in which it is stored. In pilot studies, when the albumin was not washed from the lenticule, an early inflammatory response could occur.
A LASIK flap is created, normally at 100 to 120 µm depth, and the lenticule is visually placed over the constricted pupil. Once the surgeon is satisfied with the centration of the lenticule, the flap is carefully replaced. I have found that waiting a few minutes allows the lenticule to stick well to the cornea, after which there is less chance of the lenticules moving when the flap is replaced.
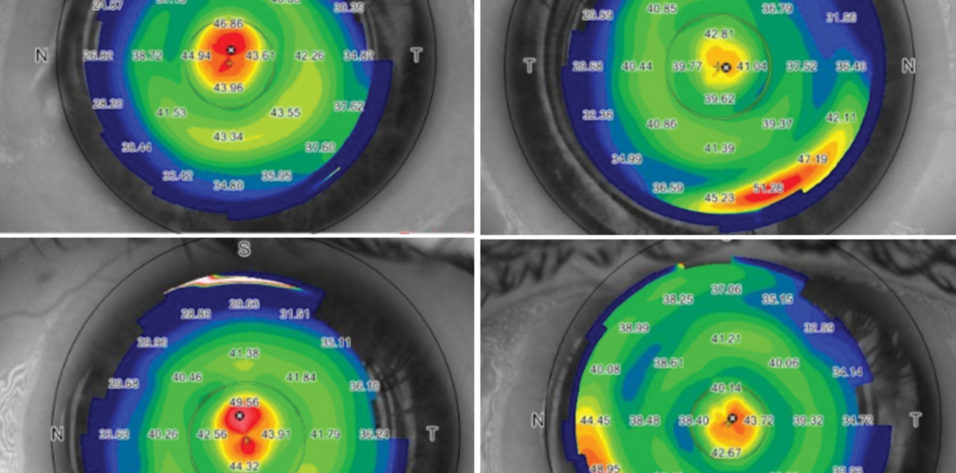
I routinely then acquire a tangential topography map with the MS39 anterior segment OCT unit (CSO Italia), which allows me to confirm the centration (Figure) and see the lenticule’s position on the OCT cross-section. The MS39 also allows the measurement of the lenticule thickness using calipers, and it has recorded thicknesses within 1 µm of the expected thickness of the in vivo lenticule.

Figure. Postoperative topographies from four patients, obtained within 10 minutes of completion of surgery, demonstrating centration of the lenticule on anterior segment OCT.
Patients are placed on a tapering dose of topical prednisolone acetate 1% ophthalmic suspension (Pred Forte, Allergan) over a 4-week period. Even though the corneas look pristine at the 1-month visit, we continue with an 8-week taper of flurometholone ophthalmic suspension 0.1% (FML, Allergan), going from four times per day down to once per day.
RESULTS
Postoperative data include visits at day 1, week 1, and months 1, 3, and 6 and will include 12- and 24-month visits when patients reach those intervals.
I have treated 15 eyes in the study to date, and my findings are encouraging. Patient satisfaction is higher than with any other corneal inlay that I have used, and UDVA is significantly better in the reading eye than it would be in a monovision patient with the same reading ability.
The Table shows the reading vision at the last visit and the trend over time in the eyes of 13 patients who have reached 3-month follow-up. All but one can read at N6 or better; the one eye that cannot has latent hyperopia of 1.50 D, which is discussed in a subsequent paragraph. All patients are very satisfied with their distance vision.

Of the three patients who have passed the 6-month mark, all have the same level of reading vision that they displayed at the 3-month postoperative visit. There seems to be no further epithelial remodeling eroding the induced spherical aberration.
No inlays have been removed. Two patients would like to change the power of their inlay if a commercial option becomes available. One of these patients had latent hyperopia that we believed was low enough (1.50 D) to allow use of the inlay, but this patient’s reading vision could be improved with a more powerful inlay. Even better, LASIK could be performed by lifting the flap and aiming for emmetropia and then replacing the lenticule. Alternatively, PRK could be performed on the flap.
The other patient was -0.50 D prior to surgery and now has too much reading power. (The patient must hold reading materials too close.) This patient requires a weaker lenticule, or, again, a simple excimer laser ablation at the same time as lenticule replacement could resolve any refractive issue.
For the study, there is only one lenticule power available (2.50 D), and the inclusion criteria have been tightened to a range of plano to 0.75 D cycloplegic refraction.
The two patients who are slightly under- or overcorrected reported that, if the only option was to remove the lenticule, both would keep it as is. The fact that it can be replaced results in high degrees of preoperative confidence on the parts of both surgeon and patient.
MORE TO COME
The study was paused after 20 eyes were enrolled to allow determination of safety and efficacy endpoints at the 1-month postoperative mark. Enrollment of the next 120 eyes has now started. Given our encouraging early results, I believe that allograft inlays may be a viable and successful corneal solution to the management of presbyopia for patients who are not ready to go the intraocular route quite yet and for patients who prefer the idea of treating one eye with an inlay rather than two eyes with presbyopia-correcting IOLs.
My early experience with the TransForm Corneal Allograft has been positive, with excellent patient satisfaction and biocompatibility. I am excited at the prospect of continuing the trial now after the mandatory pause has been completed.