CASE PRESENTATION
In the past year or so, the question that I have heard from doctors most often has gone something like this: “I have a postrefractive surgery patient on whom I performed cataract surgery, and now I have a refractive surprise. What can I do to fix it?” I have therefore devoted this and three previous installments of the case files series to answering this question with the help of several respected experts on the subject. (The earlier articles are available here: bit.ly/rscase1019A, bit.ly/rscase1019B, and bit.ly/rscase1019C.) The goal is prophylaxis. How can ophthalmologists fine-tune cataract surgery outcomes in patients with a history of refractive surgery, and how can surgeons prevent postoperative problems with quality of vision? In each case, the presumption is that no refractive information is available prior to the intervention.
In this month’s installment, a 61-year-old man presented in 2017 for a cataract surgery consultation and reported decreased visual acuity in his right eye. The patient had undergone eight-incision radial keratotomy (RK) with two astigmatic keratotomy incisions in 1992. In 2014, he underwent LASIK, followed by a PRK enhancement 1 year later.
In records from 2015, UCVA was 20/20 OD, and the patient had a manifest refraction of +0.50 +0.25 x 047º = 20/20 OD. In 2017, UCVA was 20/150 OD, and the patient wore over-the-counter reading glasses. His manifest refraction spherical equivalent in that eye was +1.50 D, and BCVA was 20/40. Glare testing produced a visual acuity of 20/50.
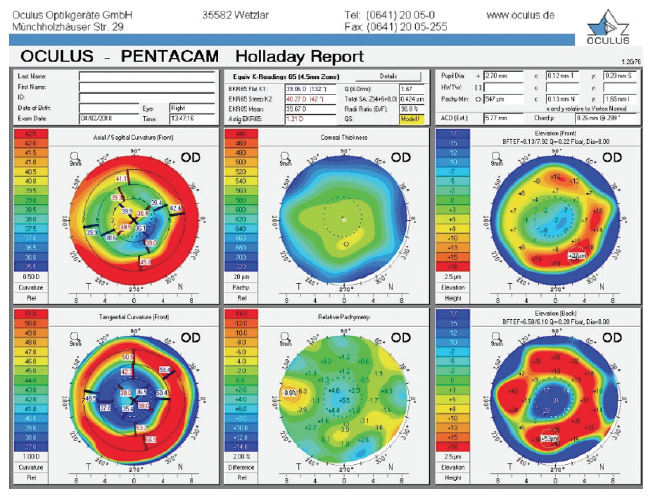
Figure 1. Holladay report on the Pentacam (Oculus Optikgeräte) prior to cataract surgery.
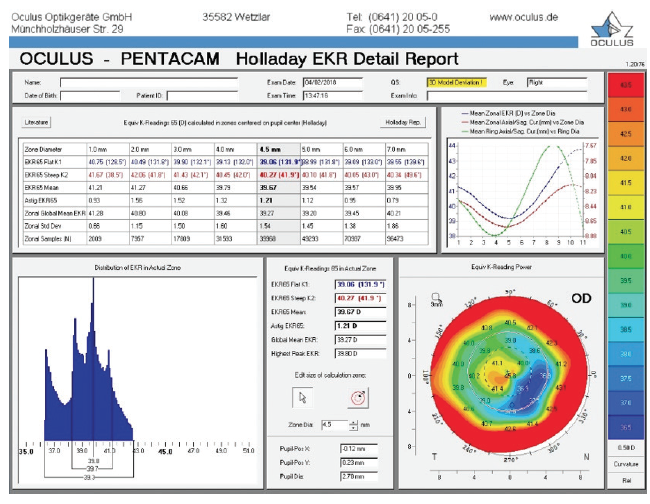
Figure 2. Holladay EKR report on the Pentacam prior to cataract surgery.
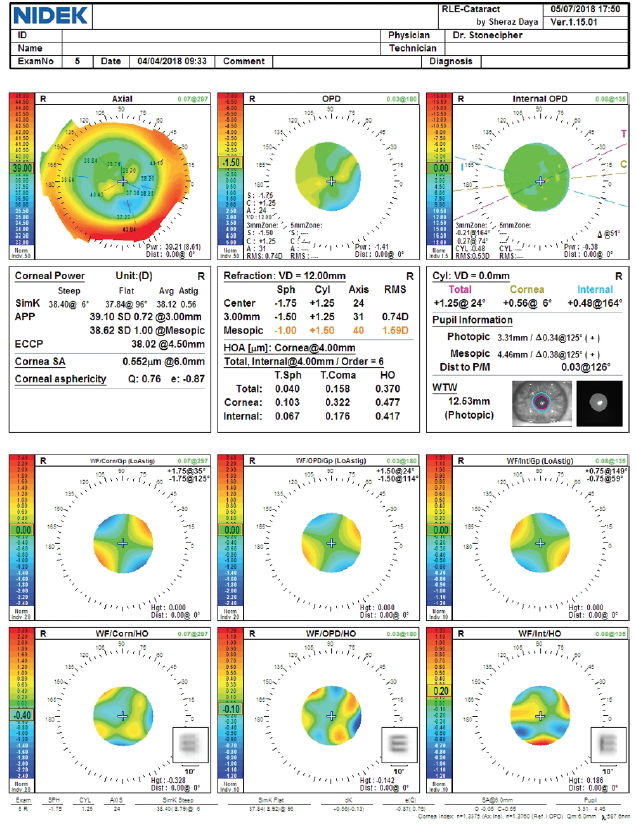
Figure 3. Preoperative measurements with the OPD-Scan III (Nidek).
During surgical planning, the target refraction was -0.50 D (Figures 1–3). Based on a combination of results using the ASCRS IOL power calculators for prior RK and prior myopic LASIK/PRK and the Barrett formula, I selected a 26.00 D Tecnis 1-piece IOL (model ZCB00, Johnson & Johnson Vision, Figures 4 and 5). I attempted intraoperative aberrometry with the ORA System (Alcon) but could not obtain a measurement.
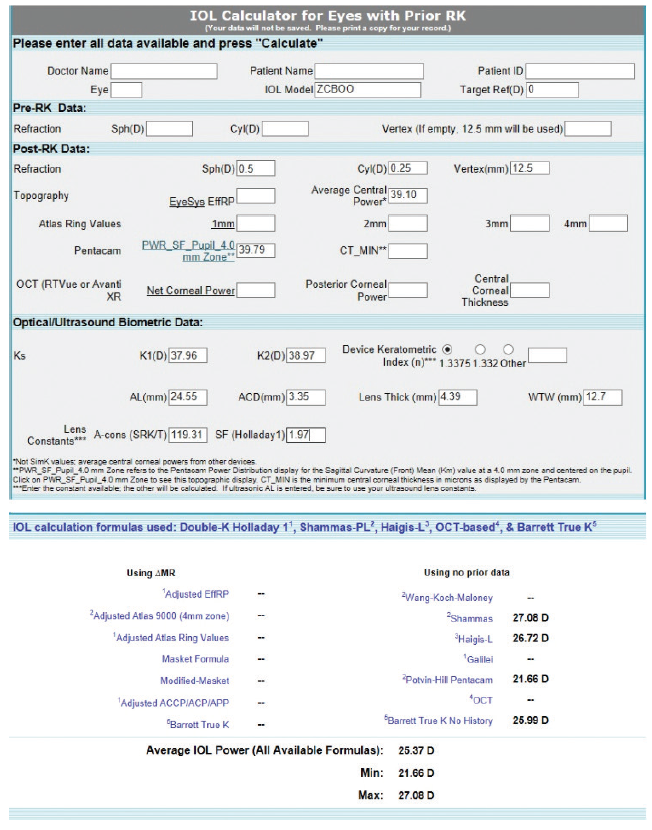
Figure 4. Preoperative calculations using the ASCRS IOL power calculator for prior myopic LASIK.
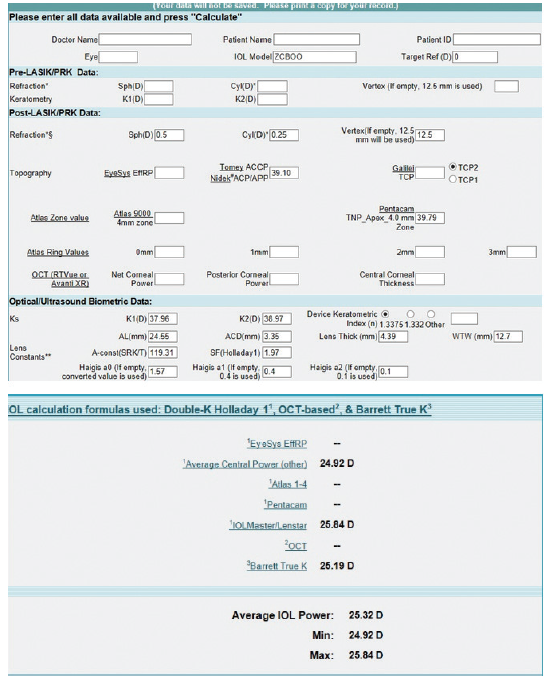
Figure 5. Preoperative calculations using the ASCRS IOL power calculator for prior RK.
Six months after cataract surgery, the patient’s UCVA was 20/60 OD. The manifest refraction was -2.00 +0.50 x 005º = 20/20.
—Case prepared by Karl G. Stonecipher, MD

BRET L. FISHER, MD
I agree with the management strategy presented. This case emphasizes the value of the surgeon’s setting realistic patient expectations because the likelihood of this patient’s needing some type of subsequent surgical intervention was high.
I would have used an average of the Barrett True K from both IOL power calculations and therefore would have implanted a 25.50 D lens, but the outcome still would have been about 1.00 D more myopic than desired. In situations like this one, many patients will accept some myopia in one eye. I would not intervene further in the right eye until a nearly plano result was achieved in the left eye—and then only if the patient could not adapt to the initially unintended monovision after a reasonable period. This may also be a case where viewing the live data stream from the ORA System could provide an estimate of the power, which could then be compared to preoperative estimates.

DAVID L. COOKE, MD
This case is ridiculously tough. It is highly unusual for a post-RK patient to need myopic treatment 12 years later. Most RK eyes become hyperopic during this time. I assume that the LASIK procedure was not to address cataract-induced myopia because the patient’s refraction gained +0.875 D in the right eye during the 2 years following PRK enhancement.
I can therefore assume that one of the following two scenarios occurred:
- No. 1: The RK treatment was minimal and thus inadequate. In this case, I would treat the eye as if it had received only myopic LASIK. The patient could likely confirm or deny this hypothesis in his history. It is unlikely.
- No. 2: The patient had hyperopic LASIK and hyperopic PRK. I like the idea of averaging the post-RK prediction with the post-hyperopic LASIK prediction for such a patient. The result achieved was close to the target.
Cataract surgery would be the patient’s fourth (refractive) operation. Preoperatively, I would stress to him that he might well need a fifth operation and that his visual acuity will likely continue to drift in a hyperopic direction.



DOUGLAS D. KOCH, MD; LI WANG, MD, PHD; AND MITCHELL P. WEIKERT, MD
We encounter patients who have undergone two or more different corneal refractive surgical procedures all too often. To calculate IOL power, the dilemma is which category of formulas to use. Which procedure has had the predominant effect on corneal curvature? Should we rely on one category of formulas (eg, post-LASIK) or pick an IOL power that is blended from two different categories of calculation methods?
This patient originally underwent eight-incision RK with two astigmatic cuts, followed by two excimer laser procedures. We do not know from the history if they were hyperopic or myopic laser ablations. We typically see patients who were progressively overcorrected by their RK procedure, leading to a hyperopic ablation. That seems to be the case here. Although the central cornea is flat overall (approximately 38.00–40.00 D), the axial maps in Figure 1 show central steepening, likely indicating a hyperopic treatment. It is noteworthy that the OPD and Pentacam images and values are different, with a simulated keratometry reading of 38.40 D and a steeper central zone on the OPD and the EKR65 of 39.67 D and decentered central steepening on the Pentacam. Welcome to the world of measuring complex corneas!
In our experience, such prominent relative central corneal steepening is uncommon in patients like this one. Normally, we use the post-RK formulas from the ASCRS website, but, in this instance, an average may be indicated. For a plano result, the prior myopic LASIK/PRK calculator gave wildly variable numbers for IOL power, ranging from 21.66 D for the Pentacam to 27.08 D for the Shammas (Figure 4). We would not recommend using this calculator for this cornea for the reasons stated earlier.
The values in the prior RK calculator were more uniform, ranging from 24.92 to 25.84 D, with the Barrett calculator recommending a 25.19 D IOL to achieve a plano result (Figure 5). We also performed calculations using the prior hyperopic LASIK calculator (Figure 6). For a plano target refraction, the predicted IOL powers range from 24.86 to 25.75 D, with the Barrett True K No History method recommending a 24.86 D IOL. If we were to average the two values from the RK and prior hyperopic LASIK calculators, therefore, we would choose a 25.00 D IOL, a power that is 1.00 D lower than that of the IOL that was implanted. Parenthetically, we have not found intraoperative aberrometry to be helpful in post-RK eyes.
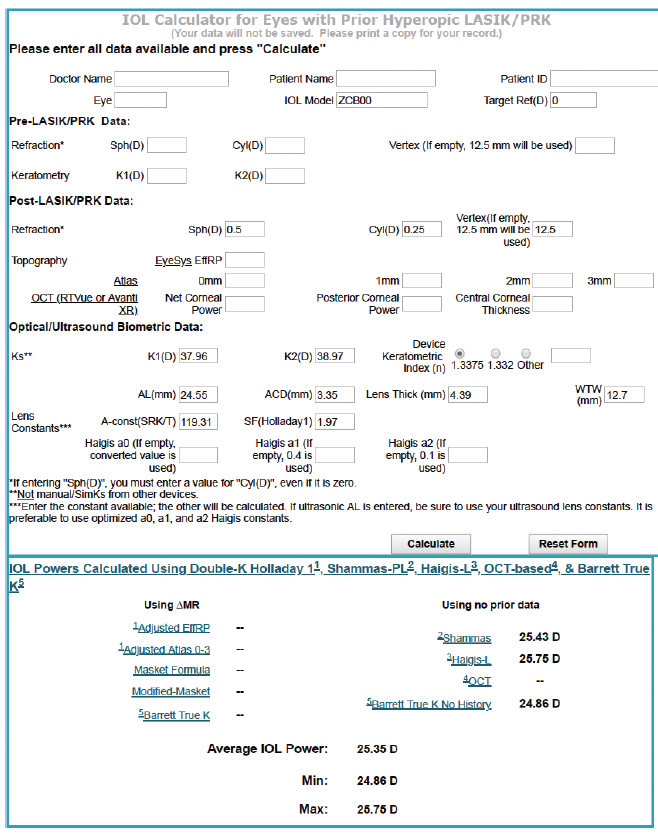
Figure 6. Calculations using the ASCRS IOL power calculator for prior hyperopic LASIK/PRK.
Courtesy of Douglas D. Koch, MD; Li Wang, MD, PhD; and Mitchell P. Weikert, MD
The spherical equivalent postoperative result was -1.75 D. If the prior hyperopic LASIK calculator and prior RK calculator results were averaged, the patient might end up with a result that was about 1.00 D more myopic than the target. That is truly the lesson of this case—that cataract surgeons are often unable to accurately calculate IOL powers for eyes such as this one. In these situations, we show patients and their families the printouts to demonstrate the high variability and the efforts we have made to try to get the result right. We inform them that an IOL exchange may be required if our calculations are off by too much.

WHAT I DID: KARL G. STONECIPHER, MD
The patient was satisfied with the near visual acuity in his right eye. For the left eye, I selected a toric IOL based on the preoperative parameters and outcomes achieved in the right eye. Despite use of dynamic intraoperative aberrometry, the outcome of cataract surgery on the left eye was unacceptable. The patient underwent an IOL exchange based on back calculations.