It’s Not About Being New, It’s About Being Better

By Allon Barsam, MD, MA, FRCOphth
What excites me most about my involvement with new IOL technologies is not that my practice offers the latest lenses, but rather being able to say that we can improve and enhance patients’ visual experiences and outcomes. I am, therefore, selective about which new IOLs I offer to my patients, and I choose only IOLs that I feel will provide them with the best opportunity to achieve the vision that they expect after cataract surgery.
Sometimes patients assume that if something is new, it must be better. But as we know from experience, that is not always the case.
THREE DECISION FACTORS
When deciding if an IOL technology is right for my practice, I first look for evidence that the new technology is safe and effective. If the IOL is based on a tried-and-true lens platform—one that has been implanted in millions of eyes—my decision is made easier, based mainly on accepting the new optical properties of the lens.
My second test for adoption of a new lens technology is to ask colleagues who have experience with that lens about patient complaints. In my opinion, a well-informed decision builds not just from published evidence—which can sometimes take many years to come to fruition—but also from user experience. It is valuable to hear from others how their patients have performed with an IOL before I decide to offer it in my practice.
It also comes down to the level of trust one has with the industry. I have worked with some IOL manufacturers who market, sell, and package their products in ways that can be confusing to both surgeons and patients. For me, therefore, trust is key—trust in your industry partner, trust in the research and development teams of that partner, and trust in its marketing team to be honest and responsible.
TRIAL PERIOD
Once an IOL technology checks those three boxes, the next step is to enter into a trial period during which we evaluate postoperative outcomes very carefully. We screen patients thoroughly and evaluate their outcomes even more carefully than we would when using a familiar lens technology.
For example, I have a lot of experience with several trifocal lens technologies, including the RayOne Trifocal (Rayner). In July 2019, I also began implanting the RayOne Trifocal Toric IOL (Figure 1). It is not routine for us to measure postoperative uncorrected and corrected intermediate vision in our trifocal patients, but we did so when we were evaluating the RayOne Trifocal Toric. We wanted to know exactly what the outcomes were in order to help us decide if the lens met our standards as a routine lens of choice.

Figure 1. RayOne Trifocal Toric IOL.
Courtesy of Rayner
I also compared results with the RayOne Trifocal Toric IOL with the results that we’ve had with other trifocal toric IOLs, including the FineVision trifocal toric (PhysIOL) and the AT LISA trifocal toric (Carl Zeiss Meditec). What I found I liked about the RayOne Trifocal Toric is that the haptics are particularly rotationally stable.
Patients want good uncorrected vision at all distances. This IOL helps patients to achieve good UCVA with minimal dysphotopsias and other aberrations. Patients rarely have any glare, although some do have more distinct halos. Halos are easier to cope with, however, because they're distinct and patients can separate them from the central image, even during activities such as nighttime driving.
CONCLUSION
New does not always mean better. It is our job as eye care practitioners to evaluate each IOL technology closely before offering it to our patients. We can do this by evaluating the clinical evidence, asking colleagues about their experiences, finding industry partners we can trust, and charting our own early outcomes closely. It is only then that we can know that this new IOL technology will make a positive impact on our patients’ overall experiences.
An EDOF-Multifocal IOL Combines the Best of Both Worlds

By Francesco Carones, MD
My boutique practice in Milan, Italy, focuses on the patient experience by providing access to the latest technologies. In Italy, cataract surgery patients who desire correction of astigmatism or presbyopia with a premium lens technology must bypass the national health system and pay for their entire surgery out of pocket. Given that routine surgery with implantation of a monofocal IOL also provides very good results—and is free under the national health system—it is mostly the prospect of spectacle independence that draws patients to a private clinic like mine.
To reach the goal of spectacle independence, we are fortunate to have at least seven different presbyopia-correcting IOLs to choose from. Each has advantages and disadvantages that must be taken into consideration to meet the specific patient’s needs and expectations. The newest lens on the market, the Tecnis Synergy IOL (Johnson & Johnson Vision), combines some of the best features of the existing bifocal and extended depth of focus (EDOF) lenses. I have implanted it bilaterally in about 15 patients so far, of whom 10 (20 eyes) have completed their 1-month follow-up examinations.
At that visit, 100% of eyes had uncorrected distance visual acuity of 0.1 logMAR or better, and 80% had 0.0 logMAR or better. When we looked at near visual acuity (30–80 cm), 100% had J2 or better, without appreciable peaks or gaps in focus. All patients reported excellent visual quality in good light conditions and being able to read as easily as they could with glasses before surgery, regardless of light conditions.
LENS DESIGN
Like the Tecnis Multifocal (Johnson & Johnson Vision), the Synergy has two distinct focal points at approximately 33 cm (near) and infinity. The Synergy also incorporates EDOF technology similar to that of the Tecnis Symfony (Johnson & Johnson Vision). In the Symfony IOL, diffractive echelette technology provides continuous vision from distance to about 60 to 65 cm; in the Synergy IOL, the EDOF portion of the lens is in the near to intermediate range, from about 33 to 80 cm.
The Synergy also filters UV and violet light, the latter of which is particularly advantageous with the rapid proliferation of LED lighting, which has gone from nearly nonexistent 10 years ago to representing more than 60% of all lighting now and is projected to account for up to 95% of the lighting market by 2025.1 LED bulbs produce a high level of light scatter due to the emission of short violet wavelengths. Blocking these wavelengths can reduce light scatter, improve contrast sensitivity, and reduce halo intensity for easier driving and device use, without blocking the healthy blue light that is needed to maintain circadian rhythm and low light vision.2,3
Like all the Tecnis lenses, the Synergy corrects for spherical aberration and is made from a material with a low rate of dispersion. The EDOF technology further corrects chromatic aberration to optimize contrast acuity, giving the Synergy the same high-quality distance vision as the Symfony or Tecnis Multifocal in bright light.
In dim light conditions, patients may notice an increase in photic phenomena at distance. I counsel patients to consider it a reasonable tradeoff for the benefits of spectacle independence. Thus far, my patients have acknowledged that they see halos but report that they are not bothered by them.
Mesopic near vision has been the weakest point for trifocal IOLs, and patients can struggle with reading in dim light. In my opinion, the pupil-independent EDOF technology in the Synergy lens makes it superior to all other current options. I don’t have to spend as much time counseling patients about not being able to read the menu in a restaurant. I also don’t need to be as careful in determining the patient’s ideal intermediate focal point (Figure 2) because the Synergy provides a continuous range of vision (Figure 3).
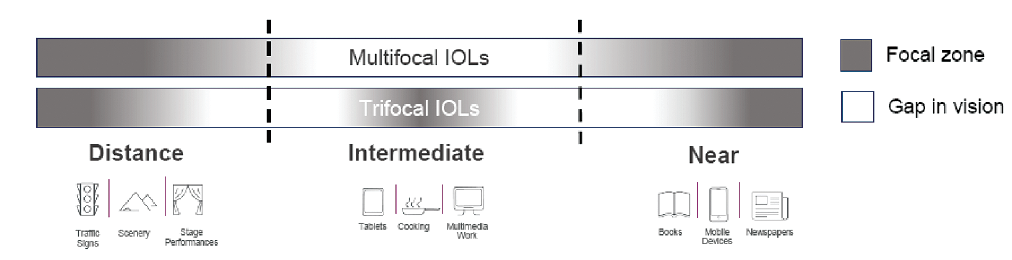
Figure 2. Bifocal and trifocal IOLs have deficits in the intermediate range.
Courtesy of Francesco Carones, MD
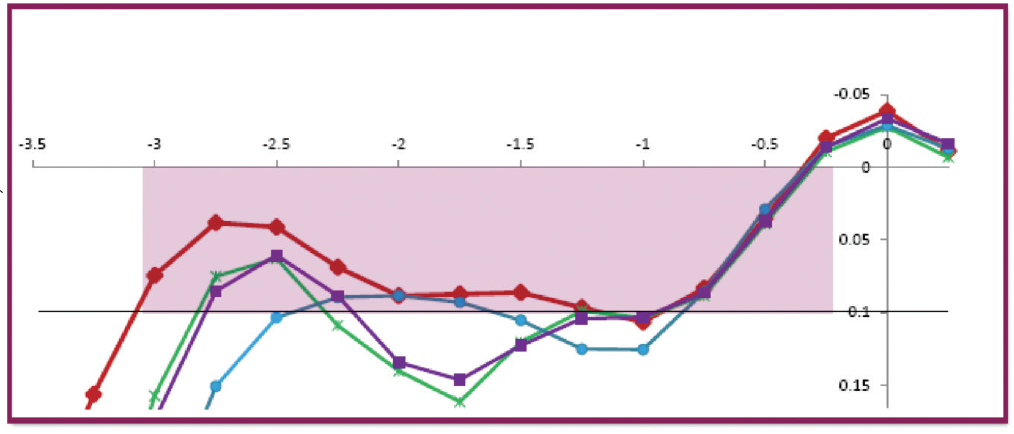
Figure 3. The Synergy IOL offers a more continuous range of vision compared to common trifocal IOLs.
Courtesy of Johnson & Johnson Vision
Of course, there are still some tradeoffs, including photic phenomena as with any multifocal IOL. Also as with other multifocal IOLs, it is essential to achieve a near-plano result. Residual refractive error, especially residual astigmatism, will degrade the visual outcome. In this sense, the Synergy is not as forgiving as the Symfony EDOF lens.
The need to achieve an emmetropic result also means that the Synergy, which is currently available only in sphere powers, is not suitable for patients with more than 0.50 D of predicted residual astigmatism. I no longer correct astigmatism on the cornea, so I opt for a toric EDOF, trifocal, or monofocal IOL in such patients instead.
CONCLUSION
Patients want good vision at all distances and in all lighting conditions. They may be willing to make tradeoffs, but they are happier if they can have it all. For patients who are willing to use low-power readers for small print, I prefer EDOF lenses because of the reduced rate of dysphotopsias. But for those who want complete spectacle independence and who don’t have much astigmatism, the Synergy IOL may be the best choice.
1. Romm J. 5 charts that illustrate the remarkable LED lighting revolution. Think Progress. August 2, 2016. https://thinkprogress.org/5-charts-that-illustrate-the-remarkable-led-lighting-revolution-83ecb6c1f472/. Accessed December 16, 2019.
2. Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784-792.
3. Mainster MA. Blue-blocking intraocular lenses: myth or reality? Am J Ophthalmol. 2009;147(1):8-10.
Complementary IOLs: A New Approach for a Continuous Range of Vision





By Jerónimo Lajara-Blesa, MD, PhD; Juan F. Zapata-Díaz, DOO, MSC, PhD; Lorenzo Vallés-San-Leandro, MD, PhD; M. Remedios Ortega-García, MD; and Miguel Ángel Rodríguez-Izquierdo, MD
The art and science of presbyopia correction are rapidly evolving to meet patient expectations. Complete restoration of the accommodative function (ie, the ability to focus at any distance) is currently not possible; however, recently developed approaches to the correction of presbyopia have been able to alleviate the symptoms to the point where patients can resume their daily activities with no impediment related to their vision. The ideal outcome for a presbyopic patient would be the complete restoration, to prepresbyopic levels, of the dioptric interval within which the patient can smoothly and rapidly achieve sharp vision.1
NO PERFECT OPTION
Bifocal, trifocal, and EDOF IOLs are well-known options to partially restore the ability to focus at several distances. The first trifocal IOLs were developed 10 years ago to overcome the need for acceptable intermediate vision, which could not be provided by bifocal IOLs.
One main limitation of multifocal IOLs on the market today is the discontinuity of their depth of focus (DOF). A noncontinuous DOF requires patients to go through a learning curve for their new way of seeing, in which only objects at certain distances (the far and near foci on bifocals, with the addition of an intermediate focus on trifocals) are seen distinctly.
In between these sharp vision foci, blur can be bothersome and incapacitating. EDOF lenses have overcome this limitation to a degree, providing an extended and continuous range of vision from far to intermediate distances, but they are unable to restore vision at near. Patients and surgeons must make a choice between a continuous DOF or near vision.
One approach to overcoming this limitation is the implantation of different addition powers or different IOL designs in a patient’s two eyes (ie, mix-and-match and modified monovision). These strategies rely on the binocular summation of images and the selection of the best image by the neural part of the visual system.
Depending on the approach used, bifocal IOLs of different addition powers (mix-and-match) have resulted in better distance corrected near visual acuity and better patient satisfaction than bilateral implantation of the same bifocal add power, without compromising contrast sensitivity, stereopsis, or visual acuity at other distances.2 Further, bilateral implantation of the same model trifocal IOL produced better intermediate and near visual acuity and contrast sensitivity than mixing and matching bifocal IOLs with different addition powers.3,4
Agreement on this approach is far from unanimous, especially because the mix does not always match the multifocal IOLs’ disparity. And discontinuity in visual acuity between far and near distances still exists throughout all these strategies.
A NEW APPROACH
An international multicenter study, with participating ophthalmologists including Tanneguy Raffray, MD, in France; Oliver Findl, MD, MBA, in Austria; and our group in Spain, is investigating an approach based on the binocular summation of images with a new set of complementary IOLs. The Artis Symbiose Mid and Plus IOLs (Cristalens Industrie) are two IOLs designed to be implanted binocularly. These preloaded one-piece diffractive IOLs employ a four-closed-loop haptic configuration and are made of a clear hydrophobic material (Figure 4).

Figure 4. The Artis Symbiose Mid (right) and Plus (left) set of IOLs.
Courtesy of Cristalens Industries
The diffractive technology used in the lens optics, based on the innovative concept of continuous phase, achieves multifocality and avoids low-contrast image gaps between the intermediate and near foci without compromising far vision. The add power continuously covers from 1.50 to 3.75 D at the IOL plane.
The Artis Symbiose Mid IOL is superior for intermediate distances, and the Artis Symbiose Plus is superior for near distances. Figure 5 shows the through-focus modulation transfer function (TFMTF) of both lenses superimposed to illustrate their complementarity.5 Note that there is a point around -2.75 D of defocus (at the IOL plane) where both TFMTF curves meet, with an MTF value of 0.15. Therefore, when the two lenses are implanted bilaterally, one of the two eyes will always have an MTF value above 0.15 that occurs between 1.50 and 3.75 D of defocus. Thus, the binocular combination of the two IOLs will provide a continuous DOF to patients from 90 to 40 cm, as used in most of the intermediate and near distance tasks of daily living, if tolerated by neural adaptation.
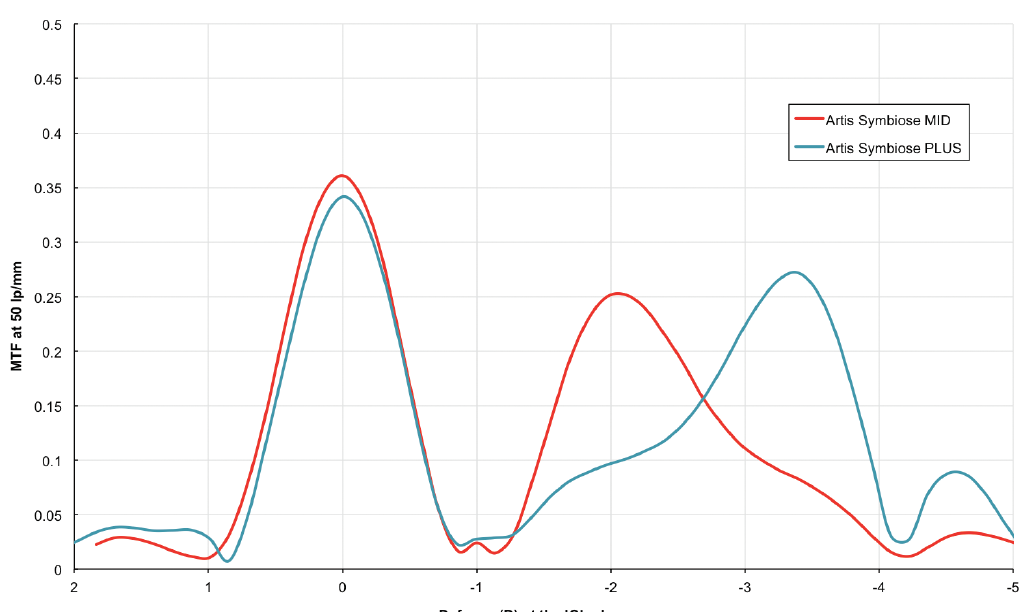
Figure 5. Through-focus modulation transfer function at 50 lp/mm for the Artis Symbiose Mid (red line) and Plus (blue line) lenses. Measurements were taken in vitro using an optical bench (PMTF, Lambda-X) and an eye model with spherical aberration to simulate that of the cornea.5
STUDY EXPERIENCE
At the beginning of 2019, the Vista Ircovisión clinics started participation in the aforementioned study to document clinical outcomes in patients implanted with the Artis Symbiose lenses. Our main purpose was to investigate the ability of this set of IOLs to provide a continuous binocular DOF from intermediate to near distances. The monocular and binocular defocus curves and contrast sensitivity measurements for the first 10 patients to reach the 1- to 2-month follow-up visit were presented at the 2019 ESCRS Annual Meeting.6
Figure 6 shows the monocular and binocular distance-corrected defocus curves for these patients under photopic conditions (~500 lux). Note that the monocular defocus curves produce a meeting point at -2.00 D of defocus at the corneal plane, similar to that of the TFMTF curves shown in Figure 5 at -2.75 D of defocus at the IOL plane.
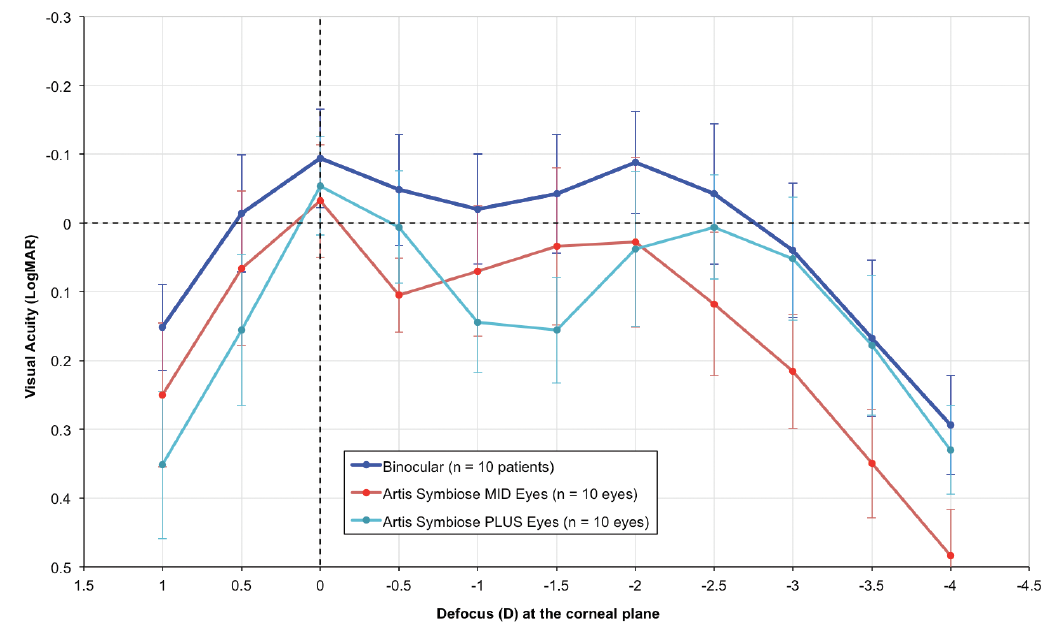
Figure 6. Distance-corrected mean monocular and binocular (dark blue line) defocus curves for the first 10 patients at 1- to 2-month follow-up. The light blue line represents the monocular defocus curve of the eyes implanted with the Artis Symbiose Plus IOL, and the red line represents that of the eyes implanted with the Artis Symbiose Mid IOL. Error bars represent ± standard deviation. Measurements were taken with an ETDRS optotype at 4 m in photopic conditions (~500 lux).6
Figures 5 and 6 courtesy of Juan F. Zapata-Díaz, DOO, MSC, PhD
The most notable observation is the binocular continuous DOF, with visual acuity better than 0.05 logMAR from 0.00 to -3.00 D of defocus at the corneal plane. There are none of the visual gaps previously observed with other lens combinations, confirming these patients’ neural adaptation to the binocular combination of the Artis Symbiose IOLs in photopic conditions.
Patient satisfaction was very high (mean score, 88.19 of 100 with the VF-14 questionnaire), and, so far, all patients are spectacle independent. Mean contrast sensitivity was inside the normal values for ages 50 to 75 years in photopic conditions.
We do not usually expect patients implanted with multifocal IOLs to be able to read immediately or soon after surgery. We were surprised to see how many patients were able to read just outside the OR after surgery. In no case did we observe a near vision learning curve. All patients could resume their daily activities immediately.
Longer-term outcomes will tell us more about the typical side effects of diffractive systems, such as dysphotopsias. Some of the patients (5 of 10) reported tolerable halos in the postoperative period. The functional burden should disappear with time, as it does for many patients with the typical diffractive trifocal and EDOF IOLs. Our ongoing study and a longer follow-up of the multicenter study will determine the answers to these questions.
CONCLUSION
We have found that the combination of the Artis Symbiose Mid and Plus IOLs can provide a continuous binocular depth of field from infinity to 33 cm in photopic conditions with no need of an adaptation period and with normal contrast sensitivity. Patient satisfaction has been high, and, so far, none of the study participants implanted with this lens combination are using spectacles.
1. Charman WN. Virtual issue editorial: Presbyopia – grappling with an age-old problem. Ophthalmic Physiol Opt. 2017;37(6):655-660.
2. Hayashi K, Yoshida M, Hirata A, Yoshimura K. Short-term outcomes of combined implantation of diffractive multifocal intraocular lenses with different addition power. Acta Ophthalmol. 2015;93(4):e287-e293.
3. Bilbao-Calabuig R, Gonzalez-Lopez F, Amparo F, Alvarez G, Patel SR, Llovet-Osuna F. Comparison between mix-and-match implantation of bifocal intraocular lenses and bilateral implantation of trifocal intraocular lenses. J Refract Surg. 2016;32(10):659-663.
4. Yesilirmak N, Akova YA, Donmez O. Comparison of mix-and-match implanted bifocal IOLs and bilateral implanted trifocal IOLs after femtosecond laser-assisted cataract surgery. J Refract Surg. 2019;35(9):559-564.
5. Zapata-Díaz JF, Lajara-Blesa J, López-Gil N. Optical performance of 4 advanced IOLs. Paper presented at: the ESCRS Annual Meeting; September 14-18, 2019; Paris.
6. Ortega-García MR, Zapata-Díaz JF, Vallés-San-Leandro L, Rodríguez-Izquierdo MA, Lajara-Blesa J. Clinical experience at Murcia with new complementary IOLs with advanced diffractive profiles. Paper presented at: the ESCRS Annual Meeting; September 14-18, 2019; Paris.
The Road to 20/20: Achieving Emmetropia With the Precisight Multicomponent IOL

By Harvey Siy Uy, MD
Are we there yet? The road to perfect vision after refractive cataract surgery has been long and filled with many twists and turns. Despite advances in biometry, IOL power calculation formulas, and IOL technologies, a significant number of eyes still develop a postoperative refractive surprise. The EuroQOL study reported that only 73% of eyes were within ±0.50 D of emmetropia after cataract surgery and, furthermore, 7% of eyes manifested greater than 1.00 D and 1% greater than 2.00 D of mean absolute error after cataract surgery.1
Even moderate amounts of refractive error produce undesired visual outcomes, especially with presbyopia-correcting IOLs. Risk factors for refractive surprise after cataract surgery include poor preoperative corrected distance visual acuity, ocular comorbidities, prior ocular surgery, and surgical complications.1 Over time, even eyes that achieved good initial refractive outcomes may undergo changes that add to the refractive error.
Methods for correcting refractive surprise include limbal relaxing incisions for postoperative astigmatism, laser refractive surgery, and implanting an add-on IOL. All of these methods entail additional costs, risks, and potential side effects. IOL exchange can correct both postoperative refractive error and multifocal optic intolerance. This method works best when performed early and may still produce uncertain outcomes due to the unpredictability of lens position after an exchange procedure.2
A novel method for correcting postoperative refractive error is the use of IOLs with exchangeable optic technologies, such as the multicomponent Precisight (InfiniteVision Optics; Figure 7) and the modular Harmoni Lens (Clarvista Medical). Both IOLs have two components: a base component that is fixed inside the capsular bag and an exchangeable optic component that can be removed any time after initial implantation and replaced with another optic with the correct dioptric power or a more acceptable optic type. Options for the latter include multifocal diffractive and extended depth of focus optics.3,4 This article focuses on the Precisight IOL.
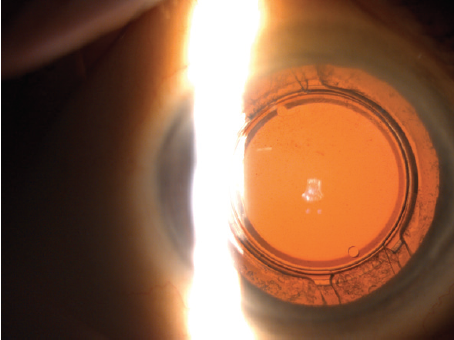
Figure 7. A slit-lamp photograph of a Precisight multicomponent IOL situated in the capsular bag after cataract surgery.
ADVANTAGES OF EXCHANGEABLE OPTIC TECHNOLOGY
The exchange process is not time-limited. My colleagues and I have found that the longer we wait, the easier it becomes to perform an optic exchange. This is because capsular fibrosis causes the base component to become fixed to the capsular bag, which provides countertraction when we remove the old optic and a stable base when we attach the replacement optic—similar to threading a thread into the eye of a unmoving needle. The fixed base of the Precisight IOL also provides excellent rotational stability after the exchange process, which is a chief requirement for toric optic use.5
Incorporating the technology into clinical practice is easy. There is no need to invest in expensive capital equipment to begin implanting Precisight IOLs. One must simply order the IOL from the manufacturer. The only difference in implanting the Precisight IOL is the additional step of attaching the two components—the hydrophobic base and the hydrophilic acrylic optic component—before loading the lens into the injection cartridge (Figure 8). The Precisight IOL can then be injected into the capsular bag just like any other IOL (Figure 9).
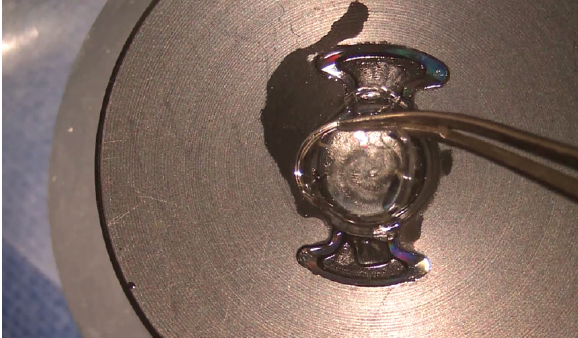
Figure 8. Operating microscope view of a Precisight IOL being assembled outside of the eye before implantation.
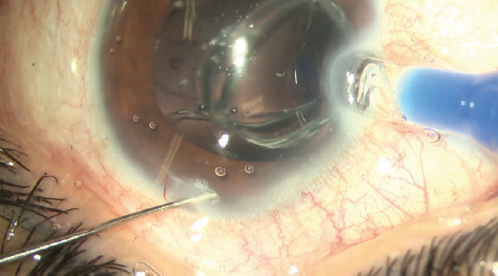
Figure 9. Operating microscope view of an assembled Precisight IOL being implanted into the eye.
REPLACING THE IOL Optic
If there is no significant postoperative refractive error, the patient has a high-quality IOL implanted into the eye with good postoperative outcomes. If there is a refractive error, I wait a few weeks and, once I am sure of the magnitude of the error, I calculate the power of the replacement IOL optic, order it, and exchange it for the original.
Removal of the optic can be achieved easily by injecting OVD through the optic dialing hole using a bent cannula, which causes the optic to lift from the base component. Then, IOL forceps are used to grasp the anchoring tab and pull the optic out through the original clear corneal incision.
Implanting the new optic is also simple. It is loaded into the same injector and inserted through the original clear corneal incision onto the base component. Standard manipulating hooks can then guide and lock the tabs of the optic into the anchoring bridges of the base component (Figure 10). No safety concerns have been observed as long as the entire multicomponent IOL has been placed into the capsular bag. Because the two components are not in contact with each other, interlenticular fibrosis does not occur.
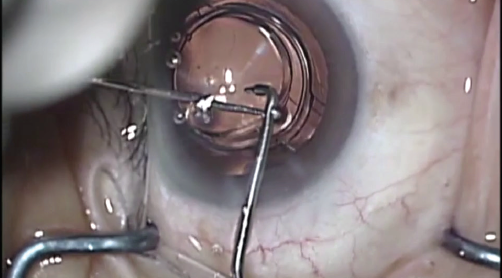
Figure 10. Operating microscope view of an exchange optic being manipulated into position during enhancement surgery.
Figures 7-10 courtesy of Harvey Siy Uy, MD
CLINICAL DATA
Data from a 6-month optic exchange or enhancement study, conducted by myself and two colleagues, showed a significant improvement of 2 lines of uncorrected distance visual acuity after enhancement surgery. It also showed a significant reduction of residual spherical equivalent (1.00 D) with a mean postenhancement surgery spherical equivalent of 0.00 D.4
No changes in pre- and postenhancement corrected distance visual acuity, anterior chamber depth, pachymetry, or keratometry were observed. A clinically insignificant decrease in endothelial cell count (2.6%; P < .01) was observed. All enhancement surgeries were uneventful, and no major complications were observed. Currently only monofocal and toric monofocal IOL optics have been implanted, but multifocal optics are under development.
CONCLUSION
Exchangeable IOL technologies are especially useful for high-risk patients, such as those with refractive error after combined phacovitrectomy, post-LASIK status, or pediatric cataracts. As we proceed on the highway to refractive perfection, exchangeable optic technologies are useful and welcome vehicles that can bring us to our destination.
1. Behndig A, Montan P, Stenevi U, Kugelberg M, Zetterström C, Lundström M. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register study. J Cataract Refract Surg. 2012;38(7):1181-1186.
2. Alio JL, Abdelghany AA, Fernández-Buenaga R. Management of residual refractive error after cataract surgery. Curr Opin Ophthalmol. 2014;25(4):291-297.
3. SooHoo JR, Lane SS, Cionni RJ, Berdahl JP, Sussman GR, Kahook MY. Comparison of stability between a modular intraocular lens system and a single-piece hydrophobic acrylic intraocular lens. J Cataract Refract Surg. 2016;42(12):1821-1825.
4. Uy HS, Tesone-Coelho C, Ginis H. Enhancement-procedure outcomes in patients implanted with the Precisight multicomponent intraocular lens. Clin Ophthalmol. 2019;13:107-114.
5. Uy HS, Tesone-Coelho C. Rotational stability of a new multicomponent intraocular lens. Clin Ophthalmol. 2019;13:1897-1907.


