As the Director General of a relatively large hospital, I believe it is my responsibility to make sure that we have the very best procedures and technologies available to our patients. Part of that responsibility, however, is also to continually evaluate those procedures and technologies that we do offer our patients. If at any time one of them is underperforming, it is my responsibility to figure out why and, if it comes down to it, decide to eliminate its use in our hospital.
Over the years, I have amassed a great deal of experience with many cataract and refractive surgery procedures, and on occasion I have had to make the tough decision to stop performing a procedure or two. Below I describe those experiences and explain my rationale for the final decision, and I also share my experience with a technology that has, in my experience, stood the test of time as an outstanding refractive surgery procedure: phakic IOL implantation.
CORNEA-BASED REFRACTIVE SURGERY PROCEDURES
In 1997, I had the privilege of performing the first LASIK procedure in Japan. Initially, I was impressed: Patients experienced rapid recovery, I could perform the procedure bilaterally in a single session, and I only had to prescribe a short use of steroids postoperatively for patients. By 2000, the procedure was approved by the Japanese Pharmaceuticals and Medical Devices Agency. But over the course of the following 10 years, patient satisfaction with LASIK declined. I noticed that some of my patients complained about their clarity of vision and some also complained of an increase in their dry eye disease (DED) symptoms. I was not alone in my observations, and various studies supported the claims that LASIK could promote tear dysfunction due to the loss of goblet cells,1 the induction of ocular surface inflammation,2,3 and the damage caused to corneal trigeminal nerves.4 By 2008, I had stopped performing LASIK and sought other methods by which I could correct my patients’ refractive errors.
In 2010, I started to perform small-incision lenticule extraction (SMILE). Again I was impressed by the procedure, but I did notice some drawbacks including a slow return to visual acuity postoperatively due to an increase in ocular scattering brought on by the procedure. As this died down over time, visual acuity gradually recovered. However, I also experienced something called interface fluid syndrome in some of my patients, which I found can occur if IOP is underestimated preoperatively. By 2015, I stopped performing SMILE.
THE PHAKIC IOL
My experience with phakic IOLs began about the same time I performed the first LASIK procedure in Japan—1997. What immediately drew me to this lens-based refractive correction approach is the fact that it is removable (and therefore reversible) and also that it seemed to provide patients with visual results that were as good as if not better than LASIK. On the flip side, at that time, implantation of the Visian ICL (STAAR Surgical) also required me to perform a peripherial iridotomy (PI) to restore a more natural flow of aqueous humor. This two-step procedure also came with the risk for patient discomfort, ocular bleeding, and bullous keratopathy. Another concern that I had was regarding late-onset cataract formation, which I had heard from colleagues was a possibility.
But the more I thought about and performed the procedure, the more I was convinced of its potential to provide patients with maximal postoperative results. Around 2004, I pursued the idea of incorporating a central hole in the optic of the Visian ICL. This, I theorized, would eliminate the need for a PI and also the risk for cataract formation. This hole, the KS-AquaPort (Figure 1), eventually revolutionized the way that we could perform phakic IOL implantation.
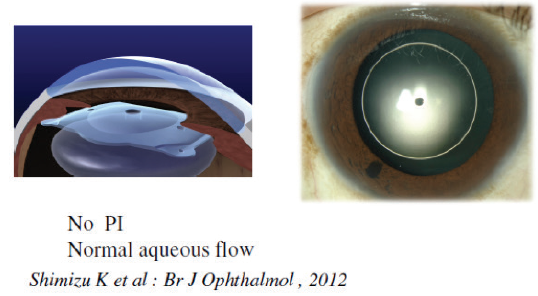
Figure 1. The EVO Visian ICL with the KS-AquaPort eliminates the need for a peripheral iridotomy.
In 2007, I implanted my first Visian ICL with the KS-AquaPort, and today I have more than 10 years of experience with this version of the lens, the EVO Vision ICL. In 2016, my colleagues and I published results of a 5-year comparative study, in which the EVO Visian ICL with the KS-AquaPort was implanted in one eye of patients and the standard Visian ICL was implanted in the other.5 Since that time, we have also populated 10-year data of all cases of EVO Visian ICL implantation performed at the Sanno Eye Center and Kitasato University Hospital from 2007 to 2017. Our results are described below.
SELECTING ICL SIZE
Historically, selection of the size of the Visian ICL (STAAR Surgical) is determined by the eye’s white-to-white or sulcus-to-sulcus measurement (Figure 1). Picking the proper size of the lens is important because it will ensure proper lens vault, which can be anywhere from 250 to 750 µm. Today, we can also use angle-to-angle measurements with an anterior segment OCT (AS-OCT) device to select ICL size. We have found this to be just as accurate if not more accurate than white-to-white measurements (Figure 2).
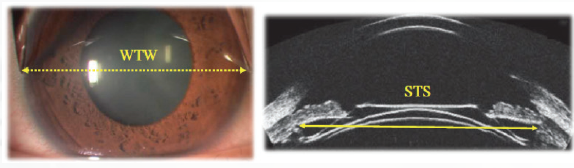
Figure 1. Historically, ICL size has been selected based on white-to-white (left) or sulcus-to-sulcus (right) measurements.
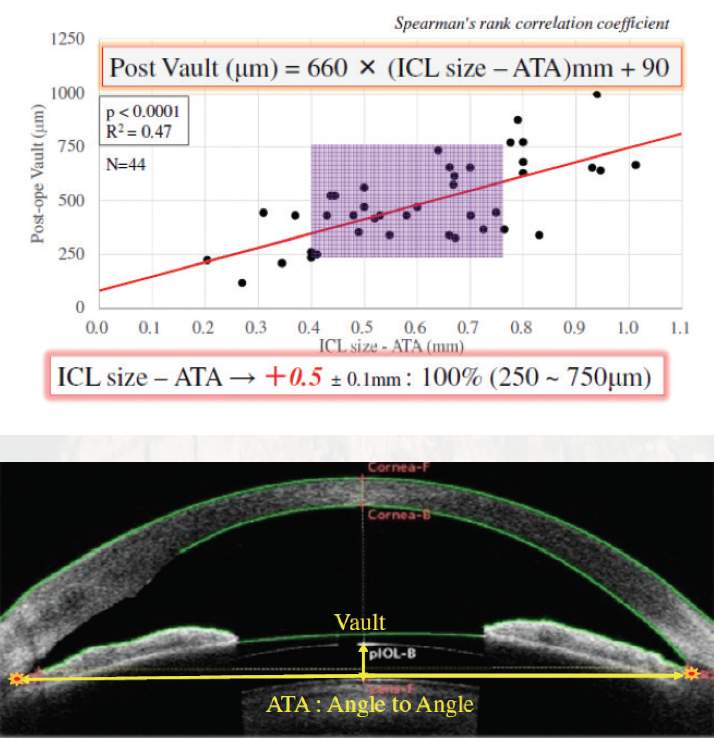
Figure 2. Another option to select ICL size is using angle-to-angle measurements as obtained by anterior segment OCT. The author has shown that angle-to-angle and vault are correlated (top). Angle-to-angle measurement with the CASIA 2 (Tomey; bottom).
As part of our long-term, 10-year study on the EVO Visian ICL, we also looked at a subset of six patients (average age 34.4 ±5.9 years) to determine if horizontal or vertical fixation was the most accurate. In each patient, horizontal fixation was achieved in one eye and vertical in the other. What we found was that the mean angle-to-angle on AS-OCT was similar in both horizontal and vertical groups (11.84 ±0.51 vs 12.10 ±0.58 mm; P <. 003). Both fixation techniques had similar safety and efficacy indices (Figure 3), and both achieved refractive predictability within ±0.50 D of intended correction in 100% of eyes.
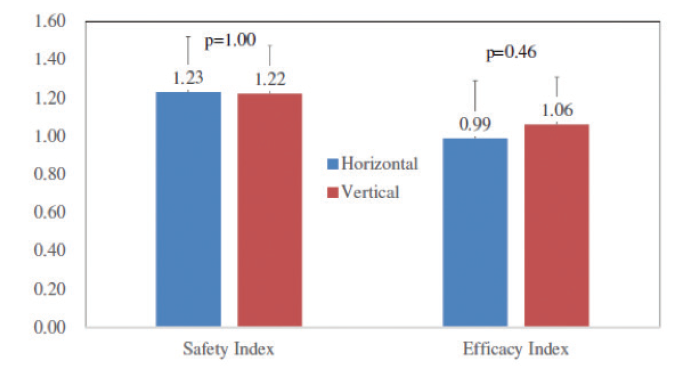
Figure 3. Safety and efficacy index for horizontal and vertical fixation of the EVO Visian ICL.
STUDY RESULTS
From 2007 to 2017, 292 EVO Visian ICLs were implanted in a total of 128 patients. The average age of patients was 34.7 ± 7.7 years (range, 20–54 years*), and the average spherical equivalent (SE) and manifest cylinder was -8.00 ±3.47 D (range, 0.50 to -23.75 D) and -1.23 ±1.26 D (range, 0.00 to -7.00 D), respectively. Of the EVO Visian ICLs implanted in this population, 129 were nontoric and 163 were toric.
A total of 130 eyes were available for 1-year follow-up. Fifty-one were available for 3-year follow-up, 34 for 5-year, 27 for 7-year, and seven for 10-year. The change in distance BCVA from 1-year to 10-year follow-up is shown in Figure 2. The change in SE from 1 week postoperative to 10 years postoperative was extremely stable, ranging from -0.20 at 1 week to -0.54 at 10 years (P = .35). Also stable was IOP, ranging from 13.4 mm Hg at 1 week to 14.2 mm Hg at 10 years (P = .56), and endothelial cell density, ranging from 2,740 cells/mm2 at 3 months postoperative to 2,673 at 10 years postoperative (P = .06).
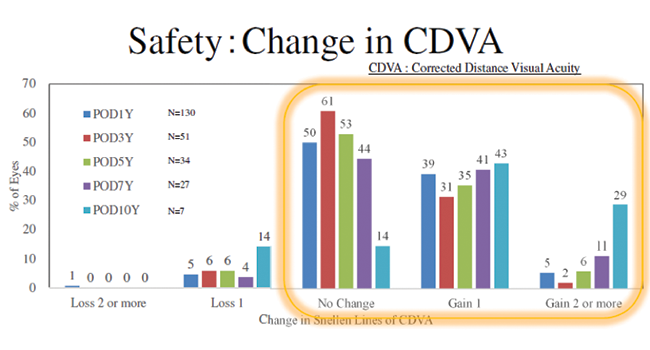
Figure 2. Change in distance BCVA from 1 year to 10 years postoperative.
As expected, there were no early term complications in any case. In long-term follow-up through 10 years, there was no incidence of infection or cataract formation, and enhancement surgery was only required in four study eyes. Of those, exchange of the EVO Visian ICL was performed in two eyes and PRK enhancement in the other two.
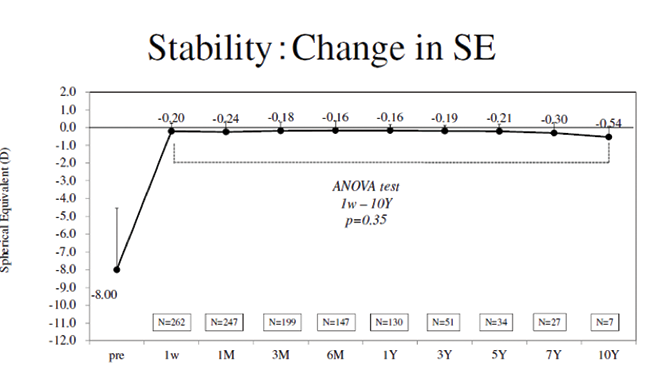
Figure 3. Change in spherical equivalent from preoperative to 10 years postoperative.
CONCLUSION
Our results indicate that implantation of the EVO Visian ICL is a safe, efficacious, highly predictable procedure that provides patients with a stable refraction over long-term use. In the 10+ years of experience with this lens at our hospitals, we have had no complications. For those reasons, the EVO Visian ICL is my first choice in refractive correction for those patients who I feel are good candidates for the procedure. I am also highly encouraged by the recent advance of selecting ICL size using OCT technology (see Selecting ICL Size).
1. Rodriguez-Prats JL, Hamdi IM, Rodriquez AE, Galal A, Alió JL. Effect of suction ring application during LASIK on goblet cell density. J Refract Surg. 2007 ;23(6) :559-562.
2. Malecaze F, Simorre V, Chollet P, et al. Interleukin-6 in tear fluid after photorefractive keratectomy and its effects o keratocytes in culture. Cornea. 1997;16(5):580-587.
3. Bilgihan A, et al. Ascorbic acid levels in human tears after photorefractive keratectomy, transepithelial photorefractive keratectomy, and laser in situ keratomileusis. J Cataract Refract Surg. 2001;27(4):585-588.
4. Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140(6):1059-1064.
5. Shimizu K, Kamiya K, Igarashi A, Kobashi H. Long-term comparison of posterior chamber phakic intraocular lens with and without a central hole (Hole ICL and Conventional ICL) implantation for moderate to high myopia and myopic astigmatism. Medicine. 2016;95(14):e3270.
*Outside the Approved age range in Japan


