CASE PRESENTATION
A 22-year-old man presented for a LASIK evaluation. A slit-lamp examination and topography measurements were normal. Pachymetry readings were 504 µm OD and 509 µm OS. The manifest refractions were -4.25 +2.00 x 090º OD and -4.50 +2.00 x 090º OS. The patient’s ocular history was significant for two contact lens–related corneal infections, one in the right eye 4 years earlier and one in the left eye 3 years earlier.
He underwent routine LASIK on both eyes using the Phorcides Analytic Engine (Phorcides) and an EX500 excimer laser (Alcon). The 100-µm flaps were created using an FS 200 femtosecond laser (Alcon) without incident or formation of an opaque bubble layer. The flaps were lifted and replaced without incident.
One day after surgery, UCVA was 20/20 OD and 20/50 OS with a bilateral UCVA of 20/20. Findings in the right eye were normal aside from inferior punctate keratopathy (Figure 1A). Grade 1 diffuse lamellar keratitis (DLK) was evident in the left eye (Figure 1B).
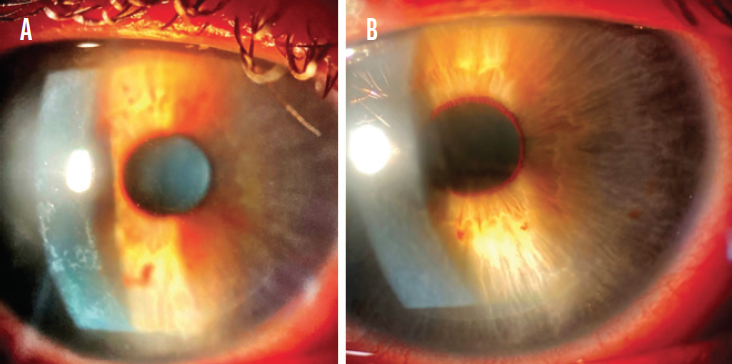
Figure 1. Inferior punctate keratopathy in the right eye (A). Grade 1 DLK in the left eye (B).
The dosing frequency for difluprednate 0.05% was increased from every 2 hours to hourly, and the patient continued to administer gatifloxacin 0.3% three times per day. The findings on examination 2 days after surgery were equivocal. On postoperative day 3, UCVA was 20/30 OS, but the DLK was grade 3 with early central mud-crack striae (Figure 2). BCVA was 20/20 OD with a plano refraction and 20/25 OS with a manifest refraction of +0.75 D.
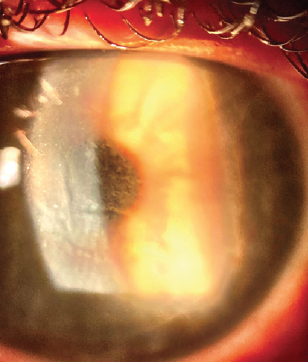
Figure 2. Centralized condensation of the DLK with mud-crack striae.
How would you proceed?
—Case prepared by Karl G. Stonecipher, MD

SIMON P. HOLLAND, MD, FRCSC, MRCP, FRCOPHTH
I would stay the course with hourly steroid treatment until day 3, when I would consider lifting the flap and irrigating the bed. This is always a difficult decision to make. Over the years, I have become increasingly reluctant to lift the flap in situations similar to this one because most of these patients do well with conservative treatment. The mud-crack striae, however, make me more inclined to lift the flap. Waiting too long to intervene risks central toxic keratopathy (CTK) with a flap melt.
I would also consider starting the patient on an oral steroid such as prednisone 50 mg administered daily for 2 days if no response was observed by day 3 or immediately postoperatively if grade 3 or more DLK was observed. The administration of a dexamethasone ophthalmic insert 0.4 mg (Dextenza, Ocular Therapeutix) could also be considered.

LOUIS PROBST, MD
Fortunately, this type of complication is exceedingly rare after LASIK. Use of a femtosecond laser rather than a microkeratome for flap creation reduces the potential for toxic contaminants to enter the flap interface. Moreover, the administration of powerful steroids such as difluprednate after LASIK helps to suppress and treat postoperative inflammation. Further, I transitioned to single-use instruments for LASIK in 2006, which eliminates the risk of autoclave-induced contamination from agents such as bacterial endotoxins.
Despite these advances and precautions, I had a case remarkably similar to this one this past year. I performed uneventful femtosecond laser–assisted LASIK on a young man. Findings were normal 1 day after surgery. On postoperative day 5, the patient returned with slightly reduced vision, a well-circumscribed area of inflammatory cells in the central interface, corneal haze measuring 2.0 x 2.5 mm, and vertical folds in the flap. The interface was irrigated, and 1 week of aggressive therapy with topical difluprednate was initiated. The folds slowly resolved over the course of 3 months, and UCVA returned to 20/20 without a hyperopic shift. In my 25 years of practice and 125,000 LASIK procedures, this was the first of my cases to fit the description of CTK.
The case presented here appears to follow the classic course of aggressive DLK. The grade 1 DLK on postoperative day 1 progressed to grade 4 by postoperative day 3 despite aggressive steroid therapy. Figures 1B and 2 show diffuse inflammation across the entire flap interface. Although differentiating between grade 4 DLK and CTK is confusing and a controversial subject, one differentiating feature is CTK’s focal, circumscribed, noninflammatory nature compared to the diffuse interface inflammation seen with DLK. Because the inflammation in this case is diffuse and progression occurred in the classic manner of DLK, I am inclined to diagnose grade 4 DLK rather than CTK, but I will be diplomatic and call it both.
Because this case is clearly associated with inflammation, I would continue aggressive treatment using a potent topical steroid such as difluprednate. Although some other surgeons would advise against it, I would also carefully irrigate the LASIK interface to reduce the number of inflammatory cells and reduce or remove the inflammatory stimulus or toxin that initiated the inflammation. The flap should be handled gently during the irrigation because the central region will be soft with loose epithelium. I would limit the steroid course to 1 to 2 weeks because the inflammation should be easily controlled by that time and the shorter course should limit the risk of an iatrogenic rise in IOP.
Patients such as this one will be appropriately concerned, so reassurance and regular monthly follow-up should be maintained until the condition resolves and UCVA returns as close as possible to the originally anticipated outcome of 20/20.

WHAT I DID: KARL G. STONECIPHER, MD
On day 3, I placed a silicone SuperEagle punctal plug (Katena) in the lower punctum of the right eye. The patient was administering difluprednate 0.05% every hour and gatifloxacin three times per day in each eye. A dexamethasone ophthalmic insert was placed in the lower punctum of the left eye. The patient was encouraged to continue therapy with difluprednate and gatifloxacin and instructed to instill nonpreserved artificial tears in both eyes in between administration of the therapeutic drops to wash out the eyes. His history was significant only in that he worked at a pet crematorium and was exposed to dusty environments and the associated chemicals.
Two weeks after the placement of a dexamethasone ophthalmic insert, UCVA was 20/20 OS, and the central cornea was clear with almost complete resolution of the mud-crack striae. Final UCVA was 20/20 OU with a plano refraction in the right eye and +0.50 D sphere in the left eye.
The following excerpt from a retrospective consecutive case series by Jutley and colleagues1 includes a summary of several published studies on CTK that are germane to this discussion:
[CTK] has been described in patients after PRK and LASIK. In a large prospective study by Stonecipher et al2 of 6,131 eyes that had LASIK, the incidence of CTK was 0.016%. The hallmark of CTK is noninflammatory opacification of the stromal bed, while the flap is spared. There is stromal tissue loss, with a consequent hyperopic shift and associated reduction in the quality of vision. In a landmark paper, Sonmez and Maloney3 postulated that the tissue loss and collapse of the central cornea was due to keratocyte apoptosis, which has subsequently been corroborated in studies using confocal microscopy to show changes in keratocytes at the affected corneal stroma under the flap.4,5 Sonmez and Maloney3 further hypothesized that the underlying etiology of CTK was a toxic reaction to a substance that undergoes photoactivation by the laser. This is an attractive theory because it might explain why the opacification is centered over the area of maximum ablation. Other theories include a toxic reaction to the powder from gloves, povidone-iodine, secretion of the meibomian glands, or postsurgical debris from the microkeratome blade.3 However, it is reasonable to say that the true causes of CTK are still unknown.
It is possible to conjecture that chemicals or aerosolized debris from this patient’s work environment could have contributed to his toxic reaction after LASIK.4-6
1. Jutley G, Aiello F, Robaei D, Maurina V. Central toxic keratopathy after laser in situ keratomileusis. J Cataract Refract Surg. 2014;40(12):1985-1993.
2. Stonecipher KG, Ignacio TS, Stonecipher KG. Complications and management with the femtosecond laser. In: Alio JL, Azar DT, eds. Management of Complications in Refractive Surgery. Springer-Verlag; 2008:169-174.
3. Sonmez B, Maloney RK. Central toxic keratopathy: description of a syndrome in laser refractive surgery. Am J Ophthalmol. 2007;143(3):420-427.
4. Hau SCH, Allan BD. In vivo confocal microscopy findings in central toxic keratopathy. J Cataract Refract Surg. 2012;38(4):710-712.
5. Thornton IL, Foulkes GN, Eiferman RA. Confocal microscopy of central toxic keratopathy. Cornea. 2012;31(8):934-936.
6. Neira W, Holopainen JM, Tervo TMT. Long-term outcome of central toxic keratopathy after photorefractive keratectomy. Cornea. 2011;30:1207-1212.



