CASE PRESENTATION
A 46-year-old man has a long history of myopic astigmatism that was successfully managed with daily soft toric contact lenses. This patient had periodically contemplated refractive surgery, but then his local ophthalmologist diagnosed him with early keratoconus during an examination.
Six months later, in February 2018, the patient presented to Duke University School of Medicine for a consultation. During this visit, he denied rubbing his eyes or sleeping in positions that might have put mechanical pressure on his eyelids. BCVA was -5.25 -0.50 x 133º = 20/20 OD and -3.75 -1.50 x 100º = 20/20 OS. Pachymetry measurements at the thinnest point of each cornea were 520 µm and 492 µm in the right and left eyes, respectively. An examination of the anterior segment of each eye was unremarkable (Figures 1–5).
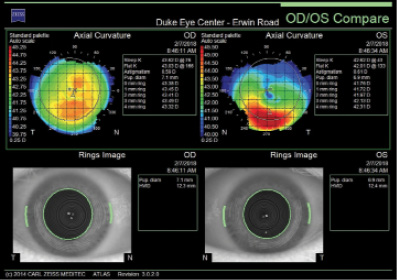
Figure 1. Curvature mapping of each eye using the Atlas 9000 Corneal Topography System at the initial consultation in February 2018.
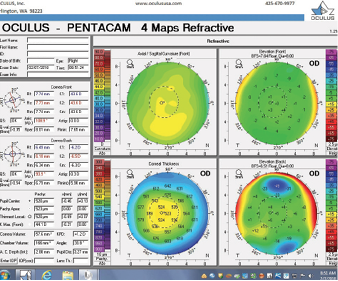
Figure 2. Tomography (Pentacam) of the right eye at the initial consultation.
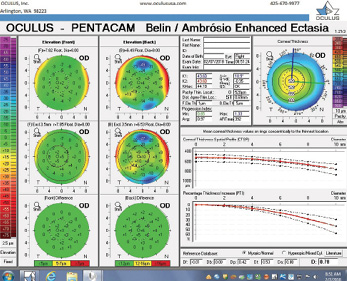
Figure 3. The Pentacam’s BAD for the right eye at the initial consultation.
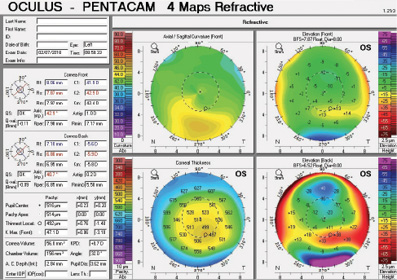
Figure 4. Tomography of the left eye at the initial consultation.
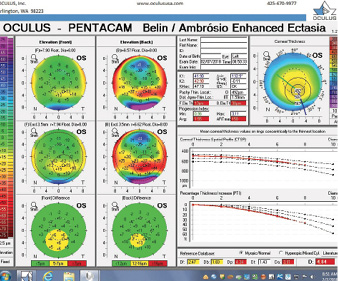
Figure 5. The Pentacam’s BAD for the left eye at the initial consultation.
One year later, the patient returned for a follow-up visit to determine whether keratoconic progression, if present, warranted intervention. He again denied rubbing his eyes. The patient stated that he had essentially given up wearing contact lenses since his previous visit, that he preferred spectacles, and that his vision was excellent. BCVA was -5.75 -0.25 x 101º = 20/20-1 OD and -3.50 -1.75 x 091º = 20/20 OS. Pachymetry measurements at the thinnest point of each cornea were 526 µm and 501 µm in the right and left eyes, respectively (Figures 6–9).
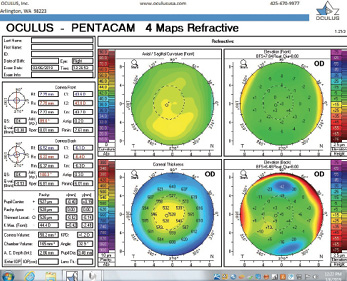
Figure 6. Tomography of the right eye during the follow-up visit in March 2019.
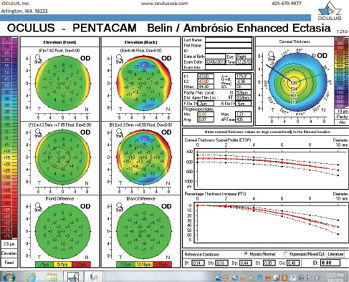
Figure 7. The Pentacam’s BAD for the right eye at the follow-up visit.
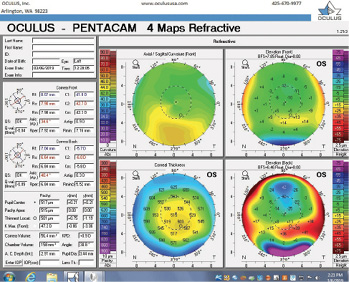
Figure 8. Tomography of the left eye during the follow-up visit.
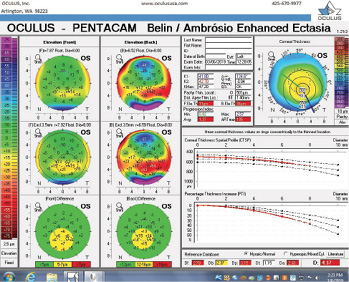
Figure 9. The Pentacam’s BAD for the left eye at the follow-up visit.
Do the findings in this case meet your criteria for keratoconus? What, if anything, would be necessary for you to make a diagnosis of forme fruste keratoconus in this case? Would you recommend intervention at this time? Regardless, what follow-up care would you suggest?
—Case prepared by Alan N. Carlson, MD

RENATO AMBRÓSIO JR, MD, PhD
Screening for ectasia risk is a major concern when we consider a patient’s candidacy for corneal laser vision correction (LVC). Enhanced screening uses a multimodal approach, including the analysis of curvature data from Placido disc–based corneal topography to detect mild ectatic corneal disease, but that’s not all.1 Our ultimate goal is to assess the cornea’s intrinsic susceptibility to ectatic progression while also evaluating the risk of biomechanical decompensation after LVC.2 Considering the significant variability in the subjective classifications of corneal topography maps,3 there is a fundamental need to acquire objective data such as the main deviation value from the Belin/Ambrósio Enhanced Ectasia Display (BAD).4,5
Corneal tomography data from the Pentacam (Oculus Optikgeräte) for this patient are available from February 2018 and March 2019 (Figure 10). Curvature data from Placido disc-based corneal topography (Atlas 9000 Corneal Topography System, Carl Zeiss Meditec) are available from the first time point, and they are identical to the topometric data obtained with rotating Scheimpflug tomography on the Pentacam. Although warpage from contact lens wear is possible, the irregular shape of the cornea in each eye had not changed over 1 year of follow-up when the patient said he did not wear contact lenses. The stability of corneal shape can be appreciated on the comparative display (Figure 10) and the Belin ABCD Progression Display (Figure 11) on the Pentacam by considering front (A) and back (B) radius of curvature at the thinnest point, thinnest value (C), and distance corrected visual acuity (D).
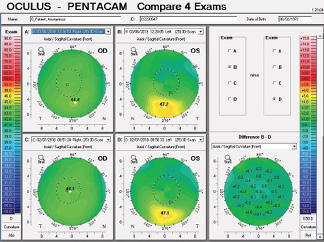
Figure 10. Comparative display of the axial front curvature from both eyes in February 2018 and March 2019.
Courtesy of Renato Ambrósio Jr, MD, PhD
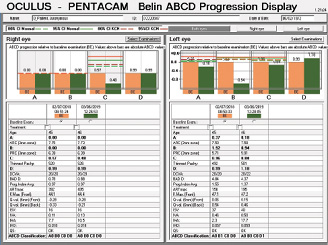
Figure 11. Belin ABCD Progression Display.
Figures 11 and 12 courtesy of Renato Ambrósio Jr, MD, PhD
The right eye has a stable front surface curvature with a relatively normal pattern despite mild inferior steepening. Figures 2 and 6 demonstrate normal and stable thickness maps and front and back elevation maps considering the 8-mm best fit sphere. The BAD is lower than 1, and the maximum Ambrósio relational thickness was greater than 390 µm at both visits. The left eye exhibits typical findings of mild keratoconus—an asymmetric bowtie and pattern of inferior steepening, which are identified as KC1 by the Pentacam’s Topographical Keratoconus Classification. Figures 4 and 8 demonstrate a coincident thinnest point and the points with highest elevation of front (15 µm) and back (42 µm) surfaces considering the 8-mm best fit sphere. Corneal tomography remained relatively stable in the left eye, such that, at both visits, the BAD was higher than 2.6 and the maximum Ambrósio relational thickness was lower than 350 µm.
A novel parameter, the Pentacam Random Forest Index (PRFI), was developed using artificial intelligence to improve detection of ectasia susceptibility based on tomographic data from a multicenter case-controlled study.6 The PRFI ranges from 0 to 1. The cutoff value of 0.22 had an area under the curve of 0.992, with 94.2% sensitivity and 98.8% specificity, this being statistically more accurate than the BAD (area under the curve = 0.960, 87.3% sensitivity, 97.5% specificity; P = .006; DeLong’s test). Interestingly, the optimized cutoff of 0.12 provided 85.2% sensitivity for the fellow eye with normal topography in eyes with very asymmetric ectasia (VAE-NT; n = 188) and 80% sensitivity preoperatively for eyes that subsequently developed ectasia after LASIK (n = 71) with 96.6% specificity.6
I was able to perform an advanced tomographic evaluation for this patient by using the raw data (u12 files) to calculate the PRFI, which was 0.11 in the right eye and 0.98 in left eye (Figure 12).
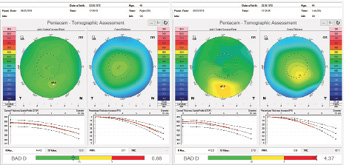
Figure 12. Advanced analysis of Pentacam data with the PRFI.
The parameters described confirm ectasia in the left eye, whereas the clinical presentation of the right eye constitutes VAE-NT. I wish to emphasize that the integration of Scheimpflug corneal tomography and corneal biomechanical data augments the accuracy of detecting subclinical ectasia; my colleagues and I found sensitivity of 90.4% and 79%, respectively, with the Tomographic and Biomechanical Index (TBI) and the BAD in 94 eyes with VAE-NT.7 For this reason, I would highly recommend using the measurement obtained with the Corvis ST (Oculus Optikgeräte) in order to calculate the TBI.
Other data that would be part of my routine evaluation are axial length, endothelial status, and ocular wavefront measurements. The results of high-definition spectral-domain OCT imaging of the cornea with epithelial mapping would also be of interest.8 The TBI and the epithelial thickness data may provide guidance on whether customized surface ablation is appropriate as well as for individualizing treatment.
Finally, considering the overall clinical data, including the level of correction and the patient’s age, custom surface ablation is an option for this patient as an elective refractive procedure. Lamellar LVC procedures such as LASIK and SMILE would not be good options due to the greater biomechanical impact. However, it is fundamental to thoroughly educate this patient about ectasia, including the need to follow up and that eye rubbing is prohibited. While I do not recommend prophylactic CXL with LVC, the patient must understand that CXL may be indicated after surgery and there may be a higher risk for ectatic progression after any form of LVC, including PRK. Considering the level of the refractive correction, implantation of a phakic IOL could also be considered. The patient must understand the lower predictability and accept the differences between refractive and therapeutic procedures.9

MICHAEL W. BELIN, MD
The examination in February 2018 found ectatic changes in the left eye with abnormalities on both the anterior and posterior cornea, abnormal pachymetric progression, and a final overall D of 4.84. These findings are definitive for keratoconus and should be considered a strong contraindication for standard refractive surgery. High asymmetry between the two eyes is also present, with a difference at the thinnest point of 28 µm. This is more than two standard deviations from the norm.10,11 Refractive evaluations exclude patients, not eyes, and I would not treat this patient regardless of the status of his right eye.
The question is what treatment, if any, should be suggested. An examination 13 months later seemingly found no significant progression, but the BAD was designed for refractive screening, not for determining ectatic progression. The newly released Belin ABCD Progression Display was specifically designed to follow ectatic change over time.12 It documents change on each anatomic level and shows when statistically significant change occurs. In the sample left eye shown in Figure 13, no ectatic progression is evident either on the anterior or posterior corneal surface or in the corneal thickness.
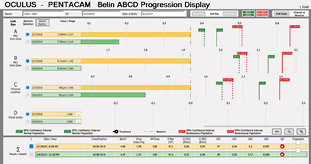
Figure 13. The Belin ABCD Progression Display graphically shows changes on the anterior (A) and posterior (B) radius of curvature taken from a 3-mm optical zone centered on the thinnest point and the thinnest corneal point (C) and distance (D) visual acuity. (Visual acuity is entered by the operator and was not entered for this patient.) This display notes whether there has been statistically significant change compared to both a normal population and a keratoconic population at both the 80% and 95% confidence intervals.
Courtesy of Michael W. Belin, MD
Based on this patient’s excellent vision, lack of documented progression, and age of 42 years, I would observe him and not consider CXL at this time. No consideration of treatment is necessary for the normal-appearing right eye, which is also stable on the progression display. I would recommend continuing to observe the patient annually. The fact that he prefers spectacles over contact lenses is advantageous because lens warpage will not be an issue in determining change if it occurs.
I do not use the term forme fruste keratoconus because I think it is useless. Historically the term described an eye that appeared normal at the slit lamp, keratometer, and optical pachymeter in a patient who had obvious keratoconus in the contralateral eye.13 The term predated modern imaging and is thus more historical than useful. Unfortunately, forme fruste keratoconus is currently used to describe a wide range of findings from normality to frank ectasia. There are even reports of bilateral forme fruste keratoconus, which by historical definition is an impossibility.

WILLIAM B. TRATTLER, MD
Unilateral keratoconus is likely common, but it often goes unrecognized. This patient, for example, was asymptomatic, his slit-lamp examination was normal, and his BCVA was 20/20 OU. He was diagnosed with keratoconus only when topography was performed to determine whether he was a candidate for refractive surgery. Placido-disc topography (Atlas 9000) and tomography (Pentacam) demonstrated a relatively normal right eye and early keratoconus in the left eye. The devices documented significant inferior steepening in the left eye. The Pentacam also demonstrated significant asymmetry between the eyes for various other parameters. The maximum keratometry reading is significantly steeper in the left versus the right eye, and corneal thickness is significantly thinner in the left eye. The thinnest point in the left eye is also displaced inferiorly, another sign consistent with keratoconus. Additionally, the left eye has an abnormal percentage thickness increase display as well as an abnormal BAD score. All of these signs support a diagnosis of unilateral keratoconus in the left eye.
On a positive note, the patient was monitored for a year, and repeated maps demonstrated stability. One way to look for subtle changes over time is a difference map, which was not provided. A difference map highlights changes in corneal shape over time. Slight steepening inferiorly and flattening superiorly (see Figure 14 for an example from a different patient) would support mild progression. Because there can be some variability from test to test, repeat testing may be performed on a different date to confirm any changes noted on a difference map.
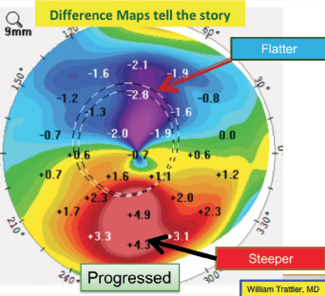
Figure 14. A difference map for another patient shows slight steepening inferiorly and flattening superiorly, suggesting progressive ectasia.
Courtesy of William B. Trattler, MD
I would educate this patient on the importance of not rubbing his eyes, and I would recommend that he return for a follow-up visit in 6 months to 1 year. The patient says that he has excellent vision, so I would not recommend CXL at this time. That said, patients in their 40s are still at risk for progression, and catching progression early allows intervention (CXL) to occur sooner.

ALAN N. CARLSON, MD
This case raises several relevant and common points of discussion, including the complexity surrounding mild corneal changes and an official diagnosis of keratoconus. I am pleased that Drs. Ambrósio, Belin, and Trattler—three of the world’s leading authorities on this topic—were willing to contribute to this article.
The term forme fruste in medicine implies a disease process that is attenuated, halted, or aborted, and it has been used to describe the unaffected or the not-yet-affected eye in a patient with so-called unilateral keratoconus. Many patients are told that there is a genetic basis for keratoconus, yet only 7% of patients with keratoconus have a positive family history of the disease. It is therefore possible that a genetic susceptibility exists in other family members who do not develop the disease process because they avoid rubbing their eyes,14,15 sleeping in positions that put pressure on their eyes, and undergoing corneal refractive surgery. My colleagues and I have also observed systemic associations with keratoconus, including a higher incidence of morbid obesity,16 floppy eyelid syndrome, and obstructive sleep apnea (floppy soft palate).17 We have recommended screening patients with keratoconus for these conditions.18
1. Ambrósio R Jr, Randleman JB. Screening for ectasia risk: what are we screening for and how should we screen for it? J Refract Surg. 2013;29(4):230-232.
2. Ambrósio R Jr, Belin M. Enhanced screening for ectasia risk prior to laser vision correction. International Journal of Keratoconus and Ectatic Corneal Diseases. 2017;6(1):23-33.
3. Ramos IC, Correa R, Guerra FP, et al. Variability of subjective classifications of corneal topography maps from LASIK candidates. J Refract Surg. 2013;29(11):770-775.
4. Ambrósio R Jr, Luz A, Lopes B, Ramos I, Belin MW. Enhanced ectasia screening: the need for advanced and objective data. J Refract Surg. 2014;30(3):151-152.
5. Ambrósio R Jr, Ramos I, Lopes B, et al. Ectasia susceptibility before laser vision correction. J Cataract Refract Surg. 2015;41(6):1335-1336.
6. Lopes BT, Ramos IC, Salomão MQ, et al. Enhanced tomographic assessment to detect corneal ectasia based on artificial intelligence. Am J Ophthalmol. 2018;195:223-232.
7. Ambrósio R Jr, Lopes BT, Faria-Correia F, et al. Integration of Scheimpflug-based corneal tomography and biomechanical assessments for enhancing ectasia detection. J Refract Surg. 2017;33(7):434-443.
8. Salomão MQ, Hofling-Lima AL, Lopes BT, et al. Role of the corneal epithelium measurements in keratorefractive surgery. Curr Opin Ophthalmol. 2017;28(4):326-336.
9. Ambrósio R Jr. Cirurgia refrativa terapêutica: por que diferenciar? Revista Brasileira de Oftalmologia. 2013;72:85-86.
10. Khachikian SS, Belin MW, Ciiolino JB. Intrasubject corneal thickness asymmetry. J Refract Surg. 2008;24(6):606-609.
11. Henriquez MA, Izquierdo L Jr, Belin MW. Intereye asymmetry in eyes with keratoconus and high ammetropia: Scheimpflug imaging analysis. Cornea. 2015;34(suppl 10):S57-60.
12. Belin MW, Meyer JJ, Duncan JK, et al. Assessing progression of keratoconus & crosslinking efficacy: the Belin ABCD Progression Display. International Journal of Keratoconus and Ectatic Corneal Diseases. 2017;6(1):1-10.
13. Belin MW, Asota IM, Ambrósio R Jr, Khachikian SS. What’s in a name: keratoconus, pellucid marginal degeneration and related thinning disorders. Am J Ophthalmol. 2011;152(2):157-162.e1.
14. Carlson AN. Keratoconus. Ophthalmology. 2009;116(10):2036-2037.
15. Carlson AN. Expanding our understanding of eye rubbing and keratoconus. Cornea. 2010;29(2):245.
16. Kristinsson JK, Carlson AN, Kim T. Keratoconus and obesity – a connection? Invest Ophthalmol Vis Sci. 2003;44:812.
17. Gupta PK, Stinnett SS, Carlson AN. Prevalence of sleep apnea in patients with keratoconus. Cornea. 2012;31(6):595-599.
18. Carlson AN. Where are the older patients with keratoconus? Cornea. 2010;29(4):479-480.



