CASE PRESENTATION
A 63-year-old woman underwent 12-cut radial keratotomy (RK) surgery in both eyes in 1990. A four-cut enhancement procedure was performed on the left eye. The patient currently has bilateral visually significant nuclear sclerotic cataracts. Salzmann nodular degeneration (SND) is present in both eyes but is more severe in the right (Figures 1–3).
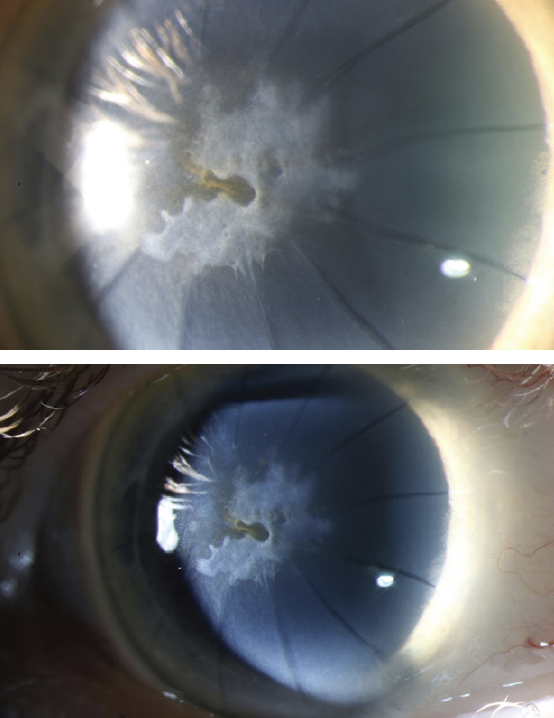
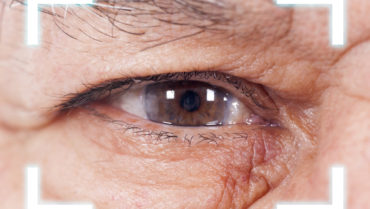
Figure 1. Visually significant SND in the right eye.
Figure 2. Anterior scarring in the left eye from SND is evident at the slit lamp.
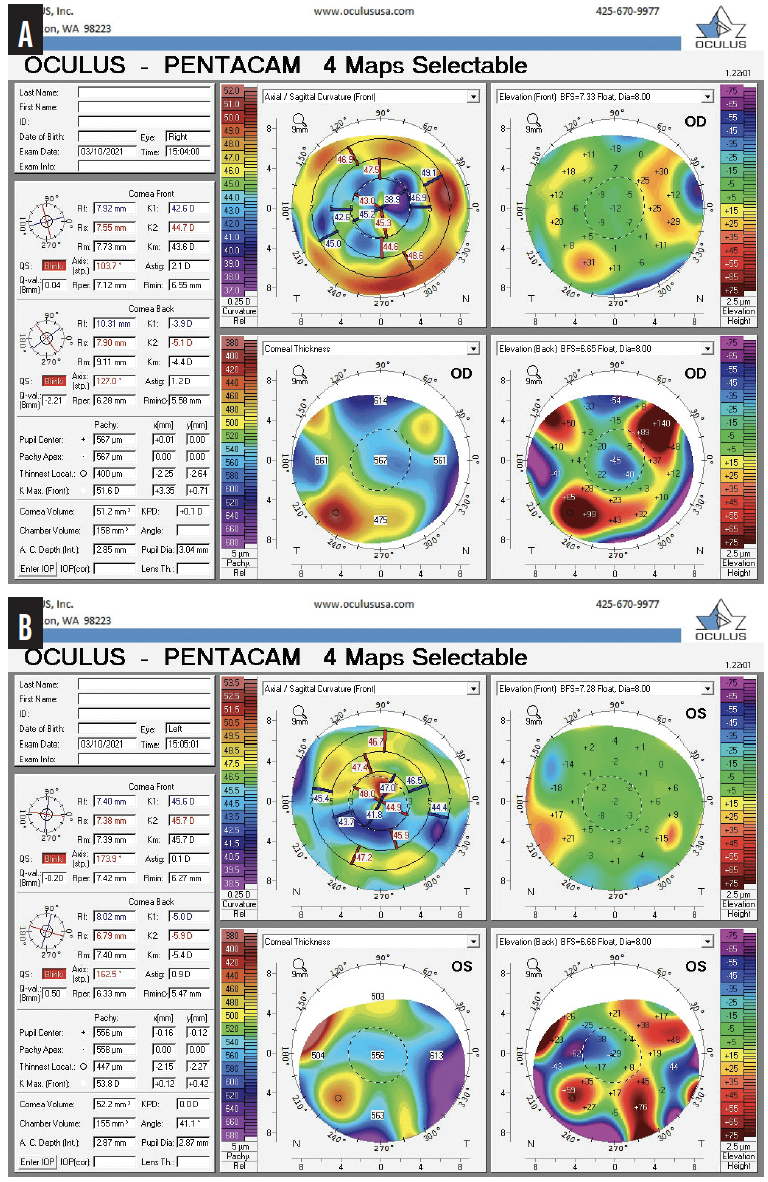
Figure 3. Imaging with the Pentacam (Oculus Optikgeräte) shows irregular astigmatism from SND and RK in the right (A) and left (B) eyes.
The patient’s ophthalmologist offered to remove the cataracts. The surgeon, however, stated that nothing can be done to address the corneal scarring and warned that this scarring will likely limit visual recovery.
Somewhat distraught, the patient visits you to request a second opinion. How would you counsel her? How would you approach surgery?
—Case prepared by Alan N. Carlson, MD

JOSÉ L. GÜELL, MD, PHD
This is a relatively common situation encountered by anterior segment surgeons—an eye with a significant corneal irregularity, more or fewer opacities, and a visually significant cataract.
To decide how to proceed, I would like to review the following information:
- The original refractive data from before RK surgery, if possible;
- BCVA with and without a rigid gas permeable (RGP) contact lens overrefraction because I find this—not tomography—to be the most practical way to evaluate the clinical impact of irregular astigmatism;
- OCT scans of the macula and optic nerve;
- Corneal high-definition OCT to evaluate the depth of the opacities and detect if an RK incision perforated Descemet layer; and
- Endothelial cell count (manual mode probably better, especially for the right eye).
Assuming that the retina and optic nerve are healthy, the approach to management depends on the degree of corneal irregularity. If it is significant (difference > 2 lines with or without an RGP lens), especially in the right eye where the opacity is more pronounced, either the patient agrees to wear RGP contact lenses after surgery or I would propose corneal transplantation (manual deep anterior lamellar keratoplasty [DALK] if no perforations are observed on the OCT scans). Obviously, if the depth of the opacity does not cross Bowman layer (Salzmann nodule), it can be removed easily. If DALK is performed, cataract surgery—or at least IOL implantation—should not occur until 2 to 3 months after full-thickness corneal surgery or anterior lamellar surgery so that keratometry readings and thus the accuracy of IOL power calculations are more accurate.
If the corneal irregularity is not significant, cataract surgery would be performed on each eye, and a monofocal IOL would be implanted in the bag. Residual myopia (-0.50 to -0.75 D) would be targeted in both eyes. Informed consent would include advising the patient that additional surgery such as laser reshaping, corneal transplantation, and placement of a piggyback IOL may be required after cataract surgery.

SADEER B. HANNUSH, MD
During the initial consultation, I would ask the patient whether she enjoyed good vision for a period of time after undergoing RK in an effort to ascertain her historical good visual potential that was later compromised. If she never enjoyed better vision than she has today, a comorbidity other than scarring may obviate the need for intervention. The preoperative evaluation should include anterior segment OCT to determine the exact location of the Salzmann-like changes (the slit image suggests the usual location anterior to Bowman layer). Other than the corneal and lenticular changes, the case presentation suggests a normal eye examination, indicating good visual potential. In the setting of irregular astigmatism, a diagnostic evaluation with an RGP lens may provide further information on the patient’s visual potential.
I would approach surgical repair in two stages. Classic Salzmann nodules peel off Bowman layer relatively easily, a procedure I routinely perform at the slit lamp. For this patient, care must be taken to avoid splaying the RK incisions, even 3 decades after their creation. Radial centripetal strokes with a crescent knife are usually effective in this situation. Phototherapeutic keratectomy with an excimer laser can be considered. In my 30+ years of experience, the changes seen in Salzmann and Salzmann-like keratopathies (as in this case) almost always are anterior to Bowman layer and can be removed with keratectomy using a crescent knife without violating the pristine Bowman layer. If, however, the keratopathy is suspected to have violated Bowman layer, as is often true in reoperated eyes, then phototherapeutic keratectomy using an excimer laser and a masking agent is the way to go.
After corneal reepithelialization, the patient would be monitored for a few weeks to confirm topographic stabilization before biometry is repeated. Any post-RK formula may be used for the IOL power calculation, but the Barrett would be my preference. It would be prudent to err toward a higher-powered IOL to avoid unwanted hyperopia postoperatively. Phacoemulsification would be performed in the usual fashion. When more than eight RK incisions are present, I prefer a posterior limbal or short scleral tunnel approach to avoid violating the RK incisions.

ERNESTO J. OTERO, MD
The surgical strategy here depends on the regularity of the cornea and the amount of haze present. Cataract surgery alone will provide a poor visual outcome even if the IOL power calculation is accurate. My first step would be corneal surgery, and DALK would be my preference. A big-bubble technique is an option; however, my experience is that, if the incisions are too deep, then the risk of perforation is high. I prefer to dissect the posterior lamella manually (Melles technique).1 Before surgery, it is important to advise patients that they will be myopic after the DALK procedure (because it removes the effect of a flattened cornea after RK) and that the improvement in vision will be minimal because of the presence of the cataract.
I would wait at least 1 month after the removal of the sutures before proceeding with cataract surgery. Phacoemulsification would be performed, and a toric monofocal IOL would be implanted to compensate for the residual refractive error. The choice of IOL depends on the amount of residual astigmatism. If it is less than 5.00 D, then any of the available toric lenses can be implanted. If a greater amount of astigmatism remains, I would choose either an AT LISA toric IOL (Carl Zeiss Meditec) or a Precizon Toric IOL (Ophtec), which can correct up to 12.00 and 10.00 D of cylinder, respectively.
This approach to rehabilitation is highly rewarding for patients in my experience, even if it takes a little longer to achieve.

KHOR WEI BOON, MBBS, FRCS(ED), FAMS
I see two options for management here, and both require some chair time to counsel the patient and set realistic expectations.
The first option is to perform a superficial keratectomy to address the SND. This is a simple, low-risk procedure. Before surgery, anterior segment OCT using the iVue80 (Optovue) would be performed to identify the depth of the nodules and detect disruption of the underlying Bowman layer. If Bowman layer has not been breached, these nodules can often be removed easily with minimal scarring using a sharp blade. Mitomycin C can be applied topically during surgery to prevent recurrence.
After the corneal surface heals and stabilizes, cataract surgery may be performed. The challenges of performing phacoemulsification on eyes with a history of RK are well documented2 and include inaccurate IOL power calculation, dehiscence of the RK incisions during surgery, and a delayed visual recovery due to a transient hyperopic shift after surgery. This approach, however, is relatively low risk, and patients generally recover reasonable vision within a short period.
The second option for intervention is DALK followed by cataract surgery. This approach may be necessary if the nodules cannot be removed adequately and if central corneal scarring, thinning, and/or irregularity remains afterward. Performing DALK in these eyes presents several risks, including dehiscence of the RK incisions during trephination and rupture of Descemet membrane if it was inadvertently penetrated by one or more RK incisions. For this reason, I perform DALK manually with a sharp crescent blade instead of using a big-bubble technique.3 If all goes well, selective suture removal for astigmatic control can begin at 6 months.
When the amount of corneal astigmatism is reasonably low and has been stable for 3 months, phacoemulsification and IOL implantation would be performed to remove the cataract and correct residual spherical error.

WHAT I DID: ALAN N. CARLSON, MD
The number of patients presenting with visually significant cataracts and concomitant scarring at the edge of a LASIK flap or in proximity to RK incisions 20 or more years after either procedure is increasing. In many instances, this scarring behaves similarly to SND, but the RK incisions or LASIK flap edge and interface necessitate a different treatment approach.
My first step was to disabuse this patient of the idea that nothing could be done to address her corneal scarring—information that greatly eased her mind. A delicate superficial keratectomy was performed to debride the cornea parallel to the RK incisions toward the center of the cornea to avoid putting traction on the RK incisions. I was prepared to perform a phototherapeutic keratectomy, but this procedure was not necessary. The ocular surface was smooth and clear, and no mitomycin C was required. A bandage soft contact lens was placed, and the patient is recovering nicely. The plan is to perform cataract surgery after her topography stabilizes.
A toric IOL is an option only if the astigmatism present is mostly regular and stable. This is more likely to be the situation when a Salzmann nodule is removed from the eye of a patient with a history of LASIK than a patient with a history of aggressive RK or one who requires lamellar keratoplasty. I would also be reluctant to implant a diffractive multifocal IOL in a patient with corneal scarring or a history of RK because the complexity of these IOL designs combined with corneal scarring can reduce the patient's quality of vision, particularly by reducing contrast sensitivity or causing glare.
Individuals who recall the success of their original refractive surgery often have high expectations for ophthalmic surgery. It generally takes additional chair time to help them understand that ophthalmic surgery at this later stage in their lives is no longer routine.
1. Melles GR, Lander F, Rietveld FJ, Remeijer L, Beekhuis WH, Binder PS. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999;83:327-333.
2. Alio JL, Abdelghany AA, Abdou AA, Maldonado MJ. Cataract surgery on the previous corneal refractive surgery patient. Surv Ophthalmol. 2016;61(6):769-777.
3. Einan-Lifshitz A, Belkin A, Sorkin N, et al. Evaluation of big bubble technique for deep anterior lamellar keratoplasty in patients with radial keratotomy. Cornea. 2019;38(2):194-197.