
Posterior capsular fibrosis is common after cataract surgery. This can be readily demonstrated through scrutiny of the large quantities of data on US Centers for Medicare & Medicaid Services (CMS) fee-for-service utilization that are available for analysis.
The graph in Figure 1 shows that, by 5 years after cataract surgery, the rate of diagnosed posterior capsular opacification (PCO) reaches almost 50%, and the rate of billed Nd:YAG laser capsulotomy treatments reaches 27%. This is based on a cohort of approximately 56,000 patients from a CMS-certified 5% statistical sample of patients who had cataract surgery in 2011 from all patients covered by CMS fee-for-service.
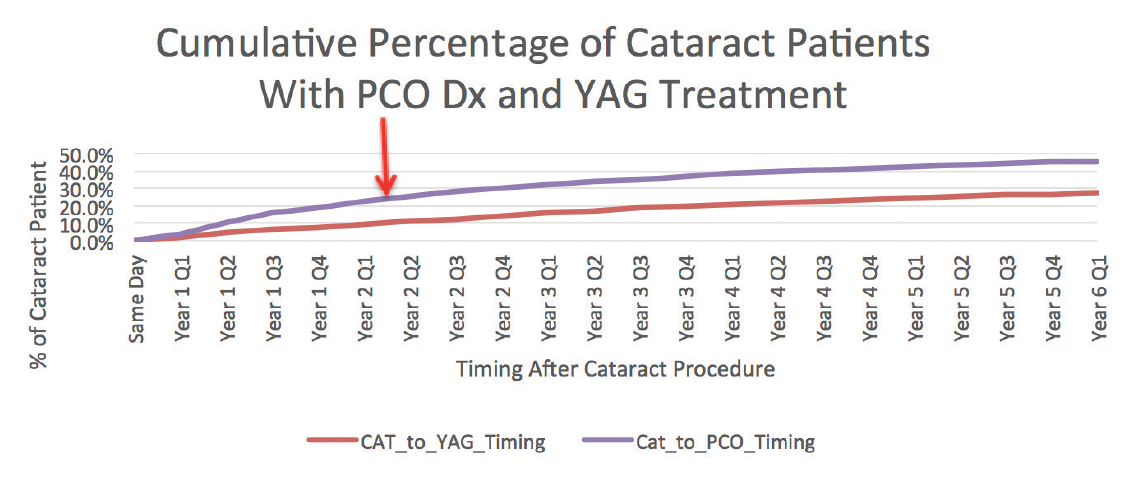
Figure 1. The cumulative incidence of PCO diagnosis and Nd:YAG laser capsulotomy in a cohort of patients who underwent cataract surgery in the CMS fee-for-service program. This graph represents the rates for a statistical sample of the 2,000,000 eyes that had cataract surgery in 2011 covered by the CMS fee-for-service program.
The most commonly implanted type of IOL in the United States in 2011 was one-piece acrylic. Therefore, the graph is generally representative of the real-world experience with modern one-piece acrylic IOLs. These rates, significantly higher than those typically reported in the peer-reviewed literature, suggest that there is more of an issue with capsular fibrosis than is commonly acknowledged.
AN INTERESTING OBSERVATION
In early 2016, I participated in initial trials of the Zepto capsulotomy system (Mynosys) at Clínica Quesada in San Salvador, El Salvador. The Zepto device was subsequently approved by the FDA in June 2017 based on a successful US-based 510(k) device clinical trial.
When we went back to El Salvador in late 2017 to follow up on the anterior capsulotomies we had created 1.5 years before, we found that they had been remarkably stable over time. But even more interesting were the posterior capsules in these eyes. There was little to no PCO at 1.5 years after surgery (Figure 2). Based on my 20 years of experience performing surgery in Central America, this absence of PCO was a very unusual finding.
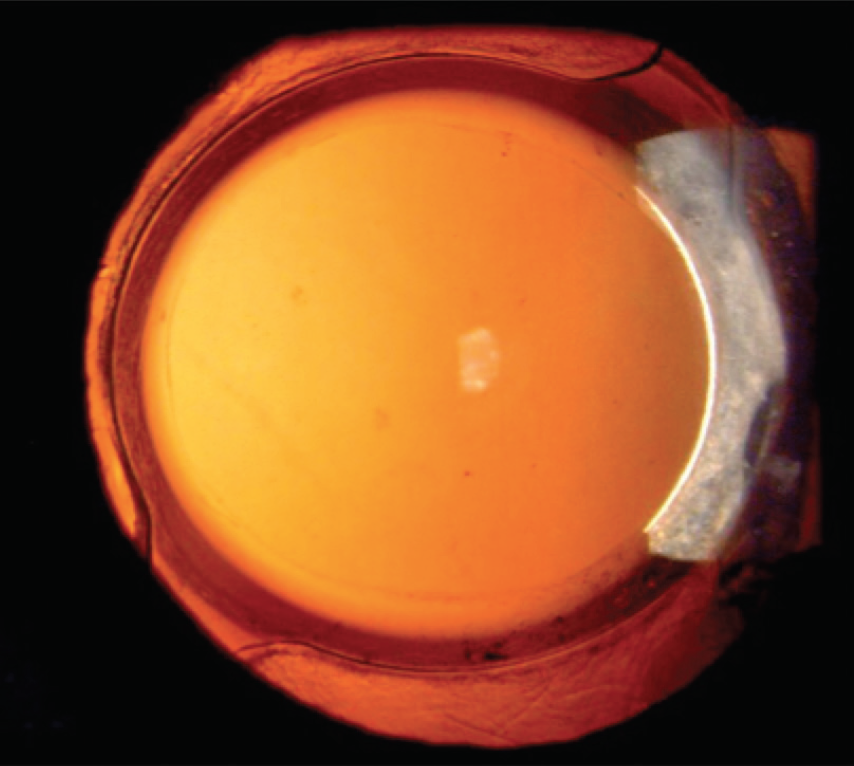
Figure 2. A patient who underwent cataract surgery in El Salvador 1.5 years earlier with Zepto capsulotomy. Note the slightly upturned capsular edge and the absence of PCO as well as the absence of fibrosis between the anterior and posterior capsules outside of the 6-mm optic.
We carefully examined the patients who had had Zepto capsulotomies approximately 1.5 years ago. Most notable was a paucity of posterior capsular fibrosis when there should have been an excess of fibrosis. This was true even outside of the 6-mm diameter of the square-edged IOLs we had used in these patients. That is, even where there was no barrier to lens epithelial cell migration and proliferation, there was little to no fibrosis. In an occasional case, we saw segmental fibrosis associated with large areas of little to no fibrosis (Figure 3).

Figure 3. Another eye from the same series. Note the typical extensive fibrosis around the haptic-optic junction and very little fibrosis elsewhere. There is little to no PCO.
FURTHER UNDERSTANDING
It is important to understand that PCO is the tip of the iceberg of capsular fibrosis and that PCO is the final common pathway of the capsular fibrosis process, not the first indication of fibrosis. By the time we see significant PCO, there is typically a large amount of fibrosis outside of the 6-mm optic.
A diagnosis of PCO indicates that there have already been large amounts of epithelial migration and fibrosis within the capsule and these have now passed the barrier function of the IOL optic.
As Figure 1 shows, approximately 50% of our routine US cataract patients are later diagnosed with PCO. This suggests that a very high percentage of eyes develops capsular fibrosis after routine cataract surgery in this country.
What we saw in the patients in El Salvador who received Zepto capsulotomies, by contrast, was little to no capsular fibrosis outside of the barrier function of the 6-mm optic. This was in an environment that is known for large amounts of capsular fibrosis. This was a completely unexpected finding.
Based on our current knowledge, we believe the strong anterior capsulotomy that the Zepto creates, combined with the flushing mechanism to release the vacuum from the eye after the capsulotomy is created, facilitates better removal of the equatorial lens epithelial cells than the typical manual capsule polishing process.
We have submitted a protocol to the US FDA to conduct a multicenter, randomized trial to confirm the ability of Zepto capsulotomy to decrease the rate of PCO. In the meantime, we hope that routine users of the Zepto device will contribute their observations regarding the possible mitigation of PCO with this device.



