
In a recently published report, Ireneusz Grulkowski, PhD, and colleagues described their new swept-source OCT (SS-OCT) system, which employs an electrically tunable lens.1 Instead of the fixed parameters of standard glass or plastic lenses, the optical properties of the new system’s lens can be dynamically controlled via an electric current (Figure 1). The single instrument is thus capable of imaging the whole eye in addition to performing quantitative optical biometry.
Figure 1. Researchers developed a new OCT system that can image the entire eye. The technology could expand the applications for OCT in ophthalmology. Pictured are Ireneusz Grulkowski, PhD (right), who led the team at Nicolaus Copernicus University, and graduate student Karol Karnowski.
(Images courtesy of Ireneusz Grulkowski, PhD)
IMAGING EVOLUTION
The first commercial ophthalmic OCT system debuted in 1996. The earliest iterations of the technology used time-domain detection, which, at 40 A-scans per second for the prototypes and 100 A-scans per second for the first commercial unit, is relatively slow at gathering information, Joel Schuman, MD, told BMC Vision. Dr. Schuman, Professor and Chairman of Ophthalmology at NYU Langone Health in New York, is one of the inventors of OCT. The first spectral-domain OCT (SD-OCT) system was introduced in 2006, and it produced more than 20,000 A-scans per second, a major leap in speed.
AT A GLANCE
- Researchers have developed a swept-source optical coherence tomography system that is capable of imaging the whole eye and performing quantitative optical biometry. In addition, the device is able to image details of the vitreous gel near the retina and crystalline lens.
- The technology could provide insight into how parts of the eye interact and the effect of opacities on visual function. This information could, in turn, lead to new applications for optical coherence tomography.
“We were able to gather much more information in the same amount of time, enabling us to do 3D OCT imaging,” Dr. Schuman said. The advance allowed users to look at volumes of tissue rather than just cross-sections. It also provided more reproducible measurements and made it possible for clinicians and researchers to detect small changes over time, he explained.
Two limitations of SD-OCT are that its resolution and sensitivity decrease with distance from the point of focus or zero delay. SS-OCT does not share these limitations, and it collects information more quickly than does SD-OCT. According to Dr. Schuman, the scanning speed of commercial SS-OCT units is approximately 100,000 A-scans per second, although he said that a speed of millions of A-scans per second is possible in the laboratory. The axial resolution of SS-OCT is not quite as high as that of SD-OCT, Dr. Schuman noted.
“With SS-OCT, we can get much denser scans in a given amount of time than with SD-OCT,” he said. “The advantages of SS-OCT allow us to think about doing things like a widefield OCT scan and not being limited, say, to a 6-mm–diameter scan.”
Today, investigators are developing new scanning patterns and new ways of looking at the retina using SS-OCT. The SS-OCT system developed by Dr. Grulkowski and his colleagues at Nicolaus Copernicus University in Torun, Poland, and Universidad de Murcia in Murcia, Spain, produces 50,000 A-scans per second (Figure 2). According to Dr. Grulkowski, its slower speed than standard SS-OCT systems allowed him and his fellow investigators to obtain “better coherence properties of the laser” and achieve “only a small signal drop through the entire depth range of the system.”
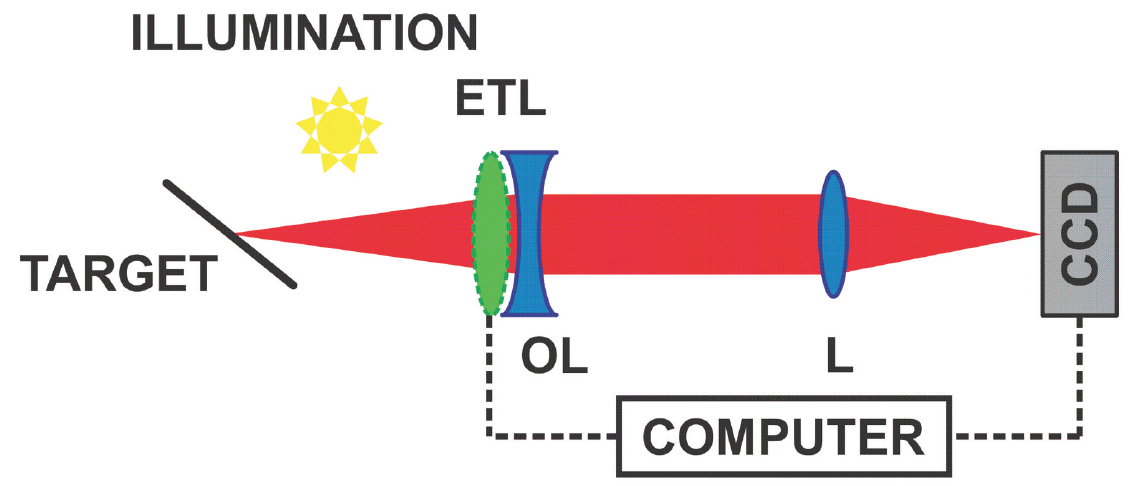
Figure 2. Light illuminates the depth-of-focus target. The electrically tunable lens (ETL) can behave as a concave or convex lens. The ETL (with an offset lens [OL]) and the lens (L) form a telescope that transforms the target plane into the plane of the camera (charge-coupled device [CCD]). Because the target is tilted, different planes are in focus during the focus tuning period, so different parts of the image are sharp at different times.
He continued, “The axial resolution of the system is 8 µm in the air. The transverse resolution depends on the configuration used. The axial resolution is comparable to [that of] regular SS-OCT systems that operate at 1 µm. Compared to SD-OCT systems that usually operate at 800 nm, the resolution is worse (SD-OCT systems can achieve 4-5 µm).”
SEEING MORE
The ability to image the entire eye will facilitate the study of how parts of the eye interact and may thus improve the applicability of OCT, Dr. Schuman remarked. Not only did the tunable lens allow high-quality imaging of both the front and the back of the eye, but also the SS-OCT system was able to image details of the vitreous gel where it interfaced with the retina and crystalline lens, Dr. Grulkowski and colleagues said. According to a January 18 press release from the journal Optica, the transparency of the vitreous gel has made this substance difficult to image, so the new technology could one day allow surgeons to use OCT to guide the removal of vitreous gel in procedures such as retinal detachment repair.
LIMITATIONS
“Current limitations of the SS-OCT system include limited speed of focus tuning,” Dr. Grulkowski told BMC Vision. “This can be overcome by the utilization of other focus tuning technology (eg, the acousto-optical tunable lenses). We would prefer also to have [a] longer imaging range. This can be obtained with the acquisition system with [a] higher sampling rate, which consequently makes aligning the subject and the imaging easier. The next challenge is also to determine and correct for distortions of the presented proof-of-concept of switching between the imaging modes. This will enable [us] to measure the shape of the eye.”
Another issue is that OCT has difficulty producing images through densely pigmented tissue such as the iris because it is a light-based technology. The view of the retina is therefore restricted by the pupil, Dr. Schuman noted.
FUTURE DIRECTIONS
Other researchers are investigating the use of OCT to measure changes in anterior lamina cribrosa depth at different IOPs as a predictive indicator of glaucomatous progression.2
Meanwhile, Dr. Grulkowski and his colleagues are working to enable their SS-OCT system to image the vitreous through its whole depth, not just near the crystalline lens and retina.
“This requires optimization of the current system,” he told BMC Vision. “This year we are planning to perform a thorough study that aims at wide-field imaging of the vitreous through its entire depth. We will try to image selected patients (eg, with floaters).”
Dr. Schuman commented that a major area of interest is OCT surgical guidance. Currently available microscopes integrated with OCT technology provide cross-sectional scans, “but a real-time 3D volume is really what we need if we are going to use the technology to a fuller level of its capability in aiding our surgical procedures,” he said.
1. Grulkowski I, Manzanera S, Cwiklinski L, et al. Swept source optical coherence tomography and tunable lens technology for comprehensive imaging and biometry of the whole eye. Optica. 2018;5(1):52-59.
2. Quigley HA, Arora K, Idrees S, et al. Biomechanical responses of lamina cribrosa to intraocular pressure change assessed by optical coherence tomography in glaucoma eyes. Invest Ophthalmol Vis Sci. 2017;58(5):2566-2577.



