
A dry eye epidemic is upon us. You can choose to recognize this and actively seek out patients to treat, or you can miss an opportunity for practice growth by ignoring it. If you look, you will find that most of your patients have dry eye disease (DED) of some stage and type. Ocular surface disease (OSD) is a complicated process involving adequate quantity and quality of tears to maintain a healthy ocular surface. Many advances have been made over the years in defining the disease, in the diagnostic tests available, and in treatment options to help patients who experience what can often be life-altering symptoms. There is still much to learn, but maybe we do not have to reinvent the wheel.
PARALLELS: DRY EYE AND GLAUCOMA
An understanding of DED is vital for cataract surgeons because achieving the best possible visual results postoperatively depends on a healthy ocular surface. The ocular surface is the first refracting surface in the eye’s ocular system, and its integrity determines the quality of subsequent refracting as incoming light proceeds to the retina.
This article presents five pearls that every cataract surgeon must know about dry eye. My approach to the topic is a little bit unusual, though, in that I discuss DED in relation to our understanding of glaucoma. Hopefully this analogy of the two diseases will help to illuminate these important points.
The philosophies used in the current understanding of glaucoma can be applied to OSD. A review of the history of glaucoma and the challenges faced in defining, diagnosing, and treating the disease may help to enhance our understanding of OSD and drive needed research.
The concept of high IOP and its relationship to glaucoma dates back to Demours in France in 1818.1 Optic neuropathy, the modern definition of glaucoma, was introduced by Drance only in 1973—155 years later.2
Korb first introduced the idea of meibomian gland dysfunction (MGD) in 1980,3 and the term meibum was coined by Nicolaides et al in 1981.4 Let us hope it does not take 155 years to achieve a better understanding of DED!
Pearl #1. KNOWLEDGE OF THE RELATIONSHIP OF STRUCTURE AND FUNCTION IN DRY EYE IS GROWING
As noted above, the scientific understanding of glaucoma has evolved, from a disease of elevated IOP to one involving optic nerve damage or optic neuropathy. The same type of evolution is taking place with DED. Through the efforts of the Dry Eye WorkShop and the International Workshop on Meibomian Gland Dysfunction, there has been a fundamental change in the understanding of the most common type of dry eye—from an aqueous deficiency to an evaporative deficiency.5,6
Diagnosis and treatment of glaucoma depends on the relationship of structure (optic nerve damage) and function (visual field constriction). This relationship determines when a patient is diagnosed with glaucoma and influences the course of treatment. Understanding of this relationship can lead to improvements in glaucoma detection and the management of individual patients.7
We are now learning more about how a structure-function relationship applies to the meibomian glands. Diagnostic testing is available to measure the structure of the meibomian glands through manual evaluation, transillumination of the lid at the slit lamp, meibography, and interferometry (Figure 1).
In early glaucoma, patients are asymptomatic while the nerve fiber layer is dropping out. In dry eye patients, the same is true. They can be asymptomatic in the early stages as the meibomian glands are slowly changing, becoming truncated, and eventually atrophying.

Figure 1. Transillumination of the lower lid. Although it is hard to photograph changes in meibomian gland structure, at the slit lamp it is easy to see gland dropout.
When is the best time to treat? Should we really be treating only once symptoms begin? In glaucoma management, that concept is long outdated. When sufficient risk factors are present, or when optic nerve damage is detected, a patient’s elevated IOP may be treated to prevent the development of visual field loss. The same may be true in dry eye; if structural changes can be detected with diagnostic technologies, DED can be treated even before it becomes symptomatic.
Pearl #2. DIAGNOSTIC TESTING ALLOWS PROACTIVE DISEASE MANAGEMENT
When you see a patient you think is at risk for glaucoma for the first time, what is your treatment plan? It is safe to assume you would schedule a return visit to start a battery of diagnostic tests to aid in the diagnosis of glaucoma, and over time you would evaluate the stability of the disease. For glaucoma suspects, the known risks associated with glaucoma guide follow-up exams.
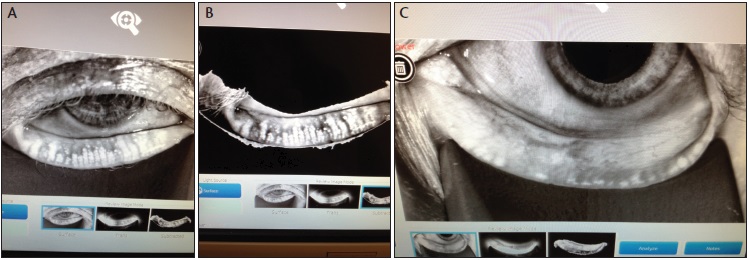
Figure 2. According to its manufacturer, the LipiView II now images meibomian glands with dynamic meibomian imaging, simultaneously employing noncontact surface illumination and high-definition transillumination to provide the most detailed gland images available (A, B). Atrophy is easily identified (C).
The same can now be done for the patient with dry eye. Gone are the days of handing an artificial tear supplement to the patient as he or she leaves your office. New treatments and diagnostic tests are available. Yes, hand the patient an artificial tear that is best suited for the appropriate deficiency, aqueous or evaporative, but also take the next step: Schedule a return visit to evaluate how it is working.
As part of that follow-up, you can start to lay the foundation from which to evaluate the effectiveness of your treatments. Does the patient have MGD? What is the appropriate approach to treatment, and how do we best monitor progress? Is there, also or instead, an aqueous deficiency? How does that factor into the equation? The field of DED and its management is still evolving, and we need to determine when and how to best test these patients and what are the gold standards for diagnosis.
Pearl #3. NUMEROUS TESTS ARE EMERGING FOR DRY EYE DIAGNOSIS
A number of structural and functional diagnostic tests have become available in recent years for DED. (All of the technologies discussed in this section are available in the US market, but their availability internationally may vary.) Still to be determined is which of these emerging dry eye tests will become the gold standards, similar to the established tests used for glaucoma patients.
In glaucoma, the standard functional test is the visual field exam. The function of the meibomian glands can be assessed with the Korb Meibomian Gland Evaluator (TearScience), which provides standardized pressure on the glands to allow objective evaluation of meibum expression.
What will become the dry eye equivalent of glaucoma structural analysis with a retinal nerve fiber layer analyzer? Meibography, introduced in 1977 by Tapie, is a method to directly visualize the meibomian gland structure.8 This technology is available on the Keratograph 5M (Oculus), and TearScience now has meibography available with the introduction of the LipiView II (Figure 2).
What about overall risk assessment, as is done in glaucoma with IOP measurement, pachymetry, and gonioscopy? Multiple diagnostics are available, including tear osmolarity measurement (TearLab), inflammatory marker detection (Inflammadry; Rapid Pathogen Screening), and interferometry with LipiView (TearScience) or TearScan (Advanced Tear Diagnostics). These tests measure either the tear film dynamics or components of the tears to evaluate for tear film dysfunction.
Pearl #4. The Basics Are Still Important
Relying solely on diagnostic tests, clinicians may either over- or underdiagnose glaucoma; the phenomenon of red disease has recently been described.9 Typically, OCT and retinal nerve fiber layer imaging devices display normal results in green and abnormal results in red. Clinicians who rely on these devices may assume that disease is present when they see a red result, but in reality the results may be due to flaws or limitations in the devices’ normative databases. There is a learning curve with any new diagnostic device, and clinicians must remember that these devices are adjuncts to clinical diagnosis.
The same can be true for DED with some of the new diagnostics described above. These tests can enable us to educate dry eye patients better, but they must be used in conjunction with good clinical evaluation.
For example, tear osmolarity is a helpful addition to aid in diagnosis and to track improvement in ocular surface health. But, much like red disease with OCT, an osmolarity number could influence the clinician to treat an evaporative dry eye patient for aqueous deficiency or vice versa. Hyperosmolar tears can be present in all types of dry eye.
Pearl #5. CLINICAL REGISTRIES CAN PROVIDE VALUABLE INFORMATION
Perhaps we need to change our thinking toward outcome-based medicine to better determine which dry eye treatments are working and to drive innovation in developing new treatments. The use of clinical registries to track and analyze patient outcomes is a relatively new concept that is gaining traction throughout medicine.
Right now, eye care providers may have no idea how effective their treatments actually are, or how what they are doing compares with what other providers are doing. That is the fundamental concept upon which outcome-based care is built. Participation in registries affords practitioners the opportunity to identify the most effective diagnostic and therapeutic processes to achieve the best outcomes for their patients. Registries can supply information that providers have never had access to before.
CONCLUSION
In the end, clinicians are all trying to improve quality of life for their patients. Research shows that both glaucoma and dry eye have negative impacts on patients’ quality of life, and both have been correlated with depression.10,11 Many of us see this firsthand, when we take the time to listen and consider the specific symptoms our dry eye patients are experiencing.
We must continue to search for answers that will further improve our understanding of DED. It is a real disease with real consequences, and it is not going away. Although some progress has been made recently, it is time that DED received the recognition, research, and collaboration among all types of eye care practitioners that it deserves.
If you have an interest or comment on the ideas in this article or an interest in participating in a dry eye registry with www.eyecareregistries.com, please contact me via email at the address below. n
1. Mantzioros N. The history of the meaning of the word glaucoma. www.glaucoma.org. Accessed November 5, 2014.
2. Morgan RW, Drance SM. Chronic open-angle glaucoma and ocular hypertension. Br J Ophthalmol. 1975;59(4):211-215.
3. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51:243-251.
4. Nicolaides N, Kaitaranta JK, Rawdah TN, et al. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20(4):522-536.
5. [no authors listed] The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5(2):75-92.
6. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-1929.
7. Sharma P, Sample PA, Zangwill LM, Schuman JS. Diagnostic tools for glaucoma detection and management. Surv Ophthalmol. 2008;53(suppl1):S17-S32.
8. Tapie R. Etude biomicroscopique des glandes de meibomius. Ann Ocul (Paris). 1977;210:637-648.
9. Chong GT, Lee RK. Glaucoma versus red disease: imaging and glaucoma diagnosis. Curr Opin Ophthalmol. 2012;23(2):79-88.
10. Kong X, Yan M, Sun X, et al. Anxiety and depression are more prevalent in primary angle closure glaucoma than in primary open-angle glaucoma [published online ahead of print November 14, 2013]. J Glaucoma.
11. Labbé A, Wang YX, Jie Y, et al. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol. 2013;97(11):1399-1403.
Leslie E. O’Dell, OD, FAAO
• The May Eye Care Center & Associates, Hanover, Pennsylvania
• helpmydryeyes@gmail.com
• Financial disclosure: None

