
Cataract surgery, specifically clear corneal phacoemulsification under topical anesthesia, is considered by many surgeons to be a routine and quick procedure with a low rate of complications. The safety of the procedure is largely thanks to adequate use of medications to prevent postoperative infection and inflammation—two potential causes of disappointing visual outcomes.
AT A GLANCE
• The advent of intracameral injections and other means of pharmacologic delivery have produced a shift toward so-called dropless cataract surgery, which aims to place less dependence on patient reliability.
• To date, the only measures shown by scientific evidence to reduce the rate of endophthalmitis after cataract surgery are preoperative use of povidone-iodine and intraoperative injection of intracameral cefuroxime.
• Subconjunctival triamcinolone seems to be less effective than topical drops at preventing CME; transzonular drug administration has some drawbacks that may limit use.
Defining the standard of care for peri- and postoperative medications in cataract surgery is a tricky matter. One can resort to national or international ophthalmic society guidelines, such as the European Society of Cataract and Refractive Surgeons (ESCRS) endophthalmitis guidelines.1 However, in the real world, we often rely on a mixture of scientific evidence and clinical experience to deliver the best possible care to our patients. Incorporating officially sanctioned guidelines into our procedure protocols could be of great help for medicolegal protection.
Until not long ago, ophthalmic surgeons relied mostly on eye drops and subconjunctival injections as the means of drug administration in relation to surgical procedures. Recently, the advent of intracameral injections and other means of pharmacologic delivery have produced a shift toward so-called dropless cataract surgery,2,3 an approach that aims to place less dependence on patient compliance or adherence to postoperative drug regimens.
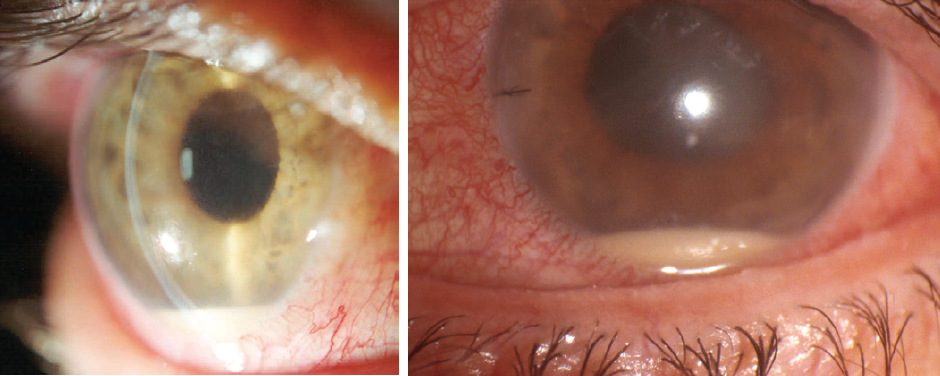
Figure 1. Postoperative endophthalmitis.
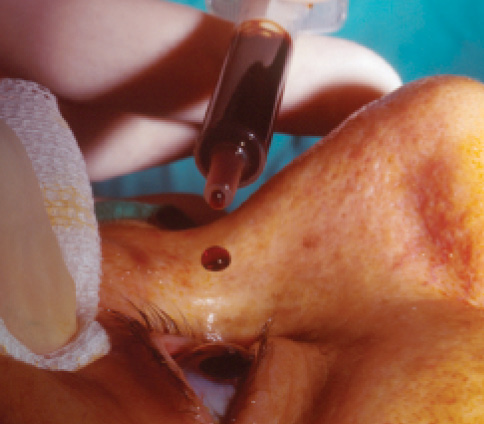
Figure 2. Topical povidone-iodine application.
INFECTION PREVENTION, PREOPERATIVE ANTISEPSIS
To date, the only measures shown by scientific evidence to reduce the rate of endophthalmitis after cataract surgery (Figure 1) are the preoperative use of povidone-iodine preparation (Figure 2) and the intraoperative administration of intracameral cefuroxime. Other measures may be useful, but it is not practical to scientifically evaluate each individual factor involved in cataract surgery.
Topical povidone-iodine, an antiseptic agent with broad-spectrum bactericidal activity, has become the standard of care for preparation of the ocular surface before cataract surgery. Its use is supported by a prospective, comparative, although not randomized, study4 and by ample clinical experience and laboratory evidence.
Different povidone-iodine concentrations and application times have been examined, but most ophthalmologists use 5% povidone-iodine applied to the cornea, conjunctival sac, and periocular skin for a minimum of 3 minutes. In a busy operating room, many surgeons are not willing to wait this long, so it may be wise to have a staff member apply an initial prep with povidone-iodine in an area outside of the operating room. In the rare instance of allergy to povidone-iodine, 0.05% aqueous chlorhexidine—an antiseptic commonly used in Sweden for cataract surgery with documented good outcomes—can be used.
ANTIBIOTICS IN CATARACT SURGERY
Preoperative topical antibiotics. Although the practice was once popular, routine use of preoperative topical antibiotics has declined. Theoretically, the bacterial load on the ocular surface would be reduced by this practice, but no study has shown that topical antibiotics effectively prevent endophthalmitis.
In high-risk patients, a short course of fusidic acid or another antistaphylococcal antibiotic plus a lid scrub seem to be reasonable prophylactic steps. Lid scrubs that contain chlorhexidine can be used, although its value for this purpose has not been properly demonstrated, and it should not be done on the day of surgery.
Although there is conflicting evidence, it is reasonable to accept that preoperative topical antibiotic use confers no additional benefit in combination with povidone-iodine, when properly used, compared with povidone-iodine alone.6 Additionally, the aim of achieving therapeutic concentrations in the anterior chamber is probably not achieved, as most of the antibiotic is washed out once surgery starts.
The findings of a large Swedish cataract registry,7 the ESCRS endophthalmitis study,8 and recent large population reports9 do not support the addition of topical antibiotics.
Intracameral antibiotics. An intracameral injection of an antibiotic at the end of cataract surgery delivers the highest and most precise dose within the therapeutic range at the site where it is needed.
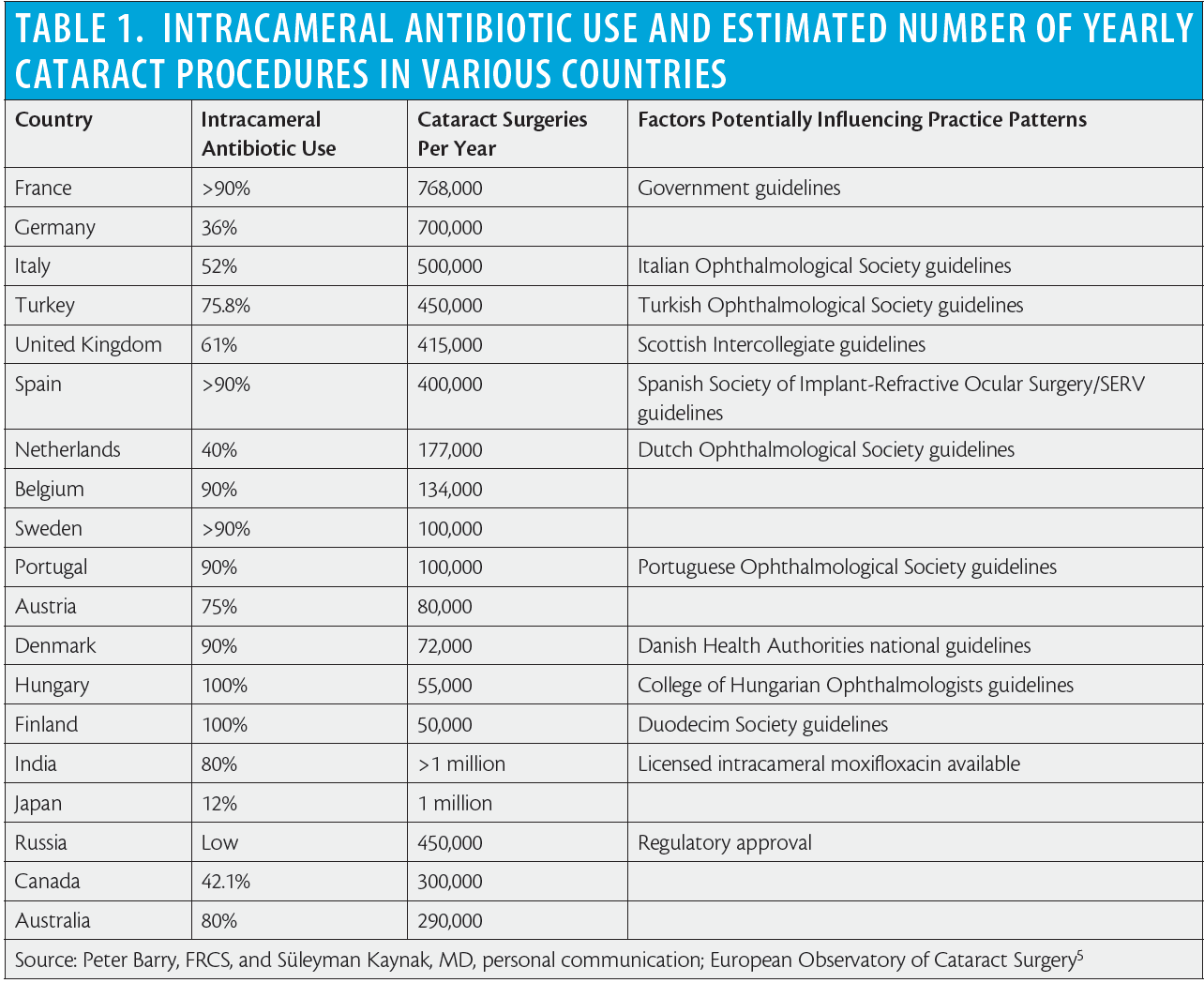
Intracameral cefuroxime use was introduced in Sweden in 1996 by Per Montan, MD, PhD, and colleagues at St. Eriks Eye Hospital. The landmark ESCRS endophthalmitis study led by Peter Barry, FRCS, scientifically proved that intracameral cefuroxime reduced the risk of contracting postoperative endophthalmitis by approximately fivefold; following that report, the adoption of intracameral antibiotics for endophthalmitis prophylaxis increased worldwide (Table 1).
American ophthalmologists have been reluctant to use intracameral antibiotics, particularly cefuroxime. According to the 2014 American Society of Cataract and Refractive Surgery (ASCRS) survey,10 only 30% of US respondents reported injecting an intracameral antibiotic.
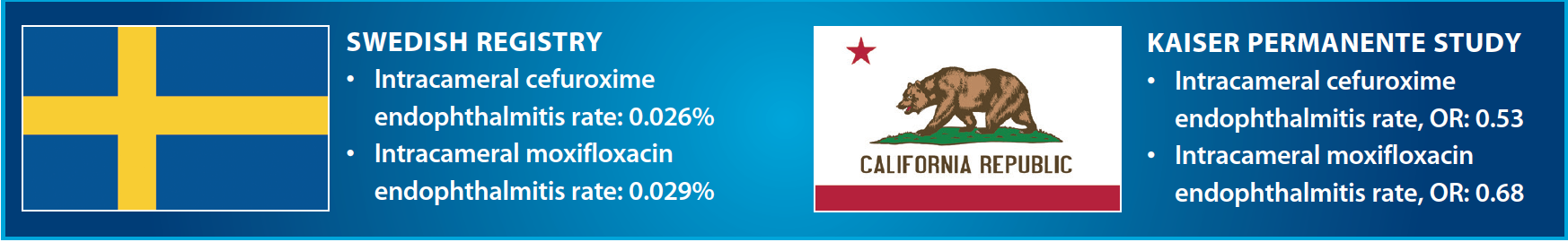
However, a recent report from practitioners with Kaiser Permanente, a large health care program in California,9 suggests that a shift in the US paradigm may be taking place. This study showed an overall endophthalmitis rate of 0.07% and an odds ratio (OR) of 0.58 (95% CI, 0.38–0.91) for the association of endophthalmitis with intracameral injection when compared with using a topical agent alone. Additionally, the combination of a topical antibiotic (gatifloxacin or ofloxacin) with an intracameral agent (cefuroxime or moxifloxacin) was not more effective than using an intracameral agent alone.
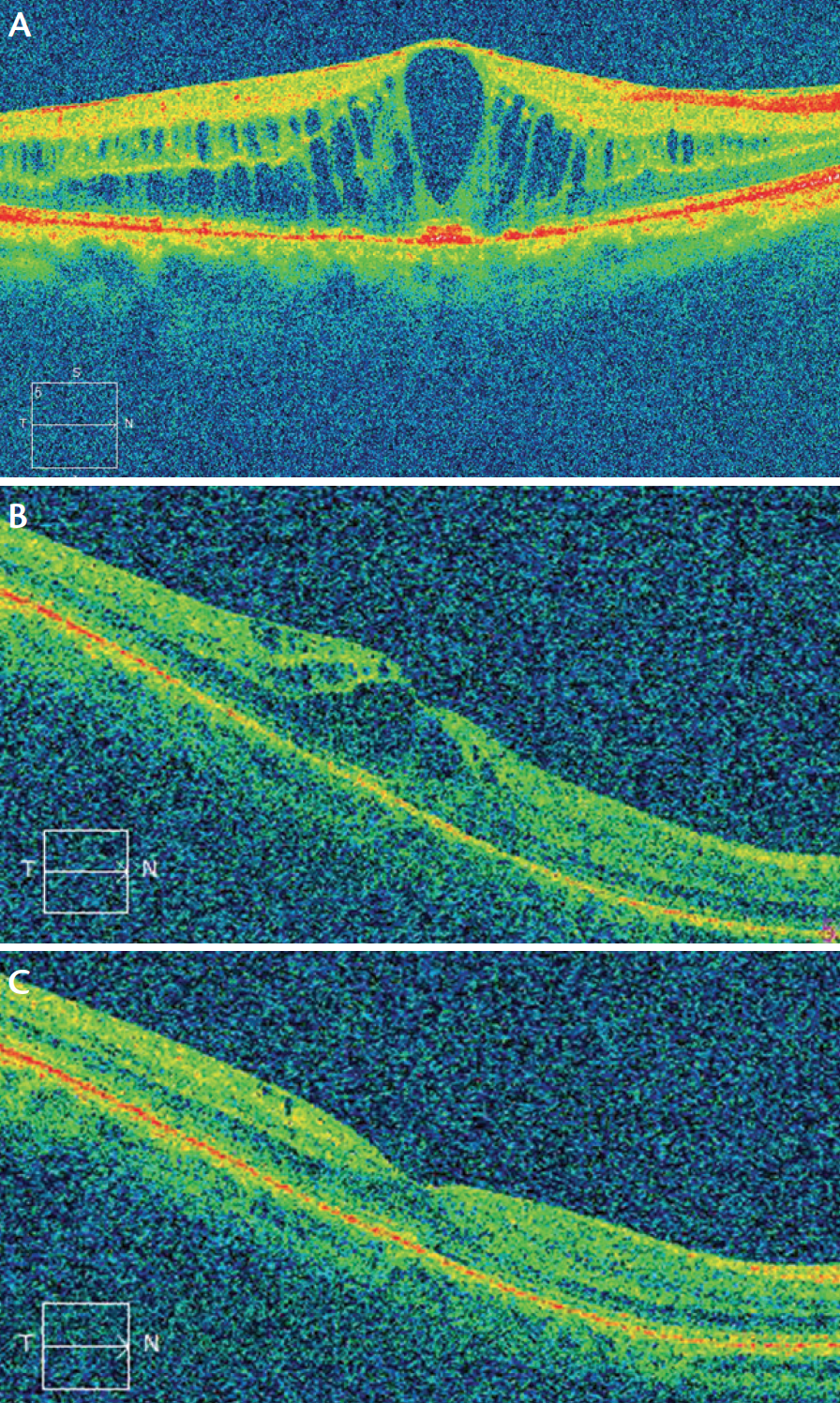
Figure 3. Postoperative CME in patients with (A) and without (B) diabetes. Resolution of postoperative CME with topical treatment in a nondiabetic patient (C).
Several conflicting editorials have recently been published in the ophthalmic literature.11-13 This fight of titans—between the antiintracameral group, led by Harry W. Flynn Jr, MD, of Bascom Palmer Eye institute, and those who consider intracameral antibiotics the way to go—may give the needed push to pharmaceutical manufacturers and the US FDA to produce and approve a licensed intracameral antibiotic preparation in the United States. Such a preparation is already available in Europe and countries in other regions.
Intracameral moxifloxacin at the end of cataract surgery has also been used, with the theoretical advantages of a broader spectrum of activity and a concentration-dependent mechanism of action. In clinical practice, the reports from both the Swedish cataract registry7 and Kaiser Permanente9 show that patients who received intracameral moxifloxacin did not have a lower rate of postoperative endophthalmitis than those who received intracameral cefuroxime.
Two factors make the option of intracameral moxifloxin less attractive: (1) the availability of a commercial preparation of cefuroxime for intraocular use in Europe and elsewhere, as opposed to use of a moxifloxacin formulation from an eye drop bottle; and (2) an increasing rate of bacterial resistance to fluoroquinolones.
Use of antibiotics in the irrigating solution is discouraged because, with this practice, not only the patient but also the staff are exposed to the antibiotic, which mostly ends up down the drain of the surgical facility. Additionally, an unknown dose is delivered in this manner, as compared with the precise amount given by intracameral injection. Use of vancomycin is particularly discouraged.
Postoperative topical antibiotics. As with preoperative topical antibiotics, there is no scientific evidence to justify the use of postoperative topical antibiotics. However, most surgeons advise their use until the cataract surgical wound has healed adequately, which is about 1 week. Retrospective studies support starting these drops early after cataract surgery,14 and this is a common approach nowadays with clear corneal phacoemulsification under topical anesthesia. It is important not to taper the drops in order to discourage the development of antibiotic resistance.
PERIOPERATIVE INFLAMMATION MANAGEMENT
Cystoid macular edema (CME) is a common postoperative complication of cataract surgery. It occurs mostly as a subclinical, self-limiting event in more than 20% of uncomplicated cases.
A recent meta-analysis15 showed that topical NSAIDs or a combination of topical corticosteroids and topical NSAIDs significantly reduced the odds of CME development after cataract surgery, as compared with topical corticosteroids alone. This suggests that a topical NSAID should always be part of the preventive treatment after cataract surgery. The conclusions of some of these studies could be questioned because less powerful topical corticosteroid formulations were used.
A large randomized clinical trial, the Prevention of Macular Edema after Cataract Surgery (PREMED) study (https://clinicaltrials.gov/ct2/show/NCT01774474), is under way with the support of the ESCRS to evaluate the effect of different preventive strategies on the occurrence of CME after cataract surgery in nondiabetic and diabetic patients. The results of this study may help clarify this situation (Figure 3).
The optimal duration of pre- or postoperative NSAID treatment is not clearly defined, but starting NSAIDs a few days before surgery reduces intraoperative miosis and seems to have a better preventive effect on postoperative CME.
As stated before, if postoperative antibiotic drops are used, they should not be tapered but rather stopped abruptly after a short course. Topical corticosteroids are usually tapered during a variable period, depending on the level of postoperative inflammation—in my experience, at around 3 to 4 weeks. NSAIDs can be stopped without tapering, also with a variable duration depending on inflammation and heightened CME risk, as in diabetic patients, uveitic patients, or complicated cases. If a longer course of NSAIDs is prescribed, the risk of corneal side effects should be assessed. For the previously mentioned reasons, we do not favor the antibiotic-corticosteroid fixed combination formulations that are popular in many countries.
CONCLUSION
In the trend toward dropless cataract surgery, traditional methods such as subconjunctival corticosteroid injection and new methods such as transzonular administration of a combination of antibiotic and steroid delivered to the anterior vitreous are intended to alleviate the need for postoperative topical medication.
Subconjunctival triamcinolone seems to be less effective than topical drops in preventing CME. Likewise, there are drawbacks associated with a subconjunctival injection in this era of topical anesthesia, and trespassing the aqueous-vitreous frontier through the zonula is uncomfortable for many surgeons. This unease may be compounded by the nuisance of anterior vitreous floaters when triamcinolone is injected. With these facts in mind, it seems that postoperative topical corticosteroids and NSAIDs are here to stay, at least for the time being.
The author would like to thank Susanne Gardner, DPharm; Per Montan, MD, PhD; Süleyman Kaynak, MD; Tat Chan, MD; and Augusto Abreu, MD, members of the ESCRS Endophthalmitis Group, for their contributions to this review. He also wishes to acknowledge the instrumental work of the late Peter Barry, FRCS.
1. Barry P, Cordovés L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas and conclusions. Paper presented at: European Society of Cataract & Refractive Surgeons Annual Meeting; 2013; Dublin, Ireland.
2. Stringham JD, Flynn HW Jr, Schimel AM, Banta JT. Dropless cataract surgery: what are the potential downsides [published online ahead of print April 6, 2016]? Am J Ophthalmol. doi:10.1016/j.ajo.2016.02.001.
3. Rhee MK, Mah FS. Cataract drug delivery systems (dropless vs. nondropless cataract surgery). Int Ophthalmol Clin. 2016;56(3):117-136.
4. Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
5. Behndig A, Cochener-Lamard B, Güell J, et al. Surgical, antiseptic, and antibiotic practice in cataract surgery: results from the European Observatory in 2013. J Cataract Refract Surg. 2015;41:2635-2643.
6. Moss JM, Sanislo SR, Ta CN. A prospective randomized evaluation of topical gatifloxacin on conjunctival flora in patients undergoing intravitreal injections. Ophthalmology. 2009;116:1498-1501.
7. Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39:15-21.
8. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicentre study and identification of risk factors. J Cataract Refract Surg. 2007;33:978-988.
9. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123:287-294.
10. Chang DF, Braga-Mele R, Henderson BA, Mamalis N, Vasavada A; ASCRS Cataract Clinical Committee. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2014 ASCRS member survey. J Cataract Refract Surg. 2015;41:1300-1305.
11. Javitt JC. Intracameral antibiotics reduce the risk of endophthalmitis after cataract surgery: does the preponderance of the evidence mandate a global change in practice? Ophthalmology. 2016;123:1411-1413.
12. Schwartz SG, Flynn HW Jr, Grzybowski A, Relhan N, Ferris FL 3rd. Intracameral antibiotics and cataract surgery: endophthalmitis rates, costs, and stewardship. Ophthalmology. 2016;123:1411-1413.
13. Olson RJ. Has the time come for all to routinely use intracameral antibiotic prophylaxis at the time of cataract surgery? Am J Ophthalmol. 2016;166:xii-xiv.
14. Wallin T, Parker J, Jin Y, Kefalopoulos G, Olson RJ. Cohort study of 27 cases of endophthalmitis at a single institution. J Cataract Refract Surg. 2005;31:735-741.
15. Wielders LH, Lambermont VA, Schouten JS, et al. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta-analysis. Am J Ophthalmol. 2015;160:968-981.
Luis Cordovés, MD
• Retina and Vitreous Section, Ophthalmology Service, Hospital Universitario de Canarias, Spain
• Member, ESCRS Endophthalmitis Study Group
• luis.cordoves@hotmail.es
• Financial interest: None acknowledged

