CASE PRESENTATION
A neuro-ophthalmologist refers a 45-year-old man for consideration of cataract surgery on his right eye. The patient was involved in an automobile accident several years ago. He states that he experienced an immediate decrease in vision in his right eye after the accident but adds that he has noticed a slow, progressive decline in the quality of his vision in that eye over the past year.
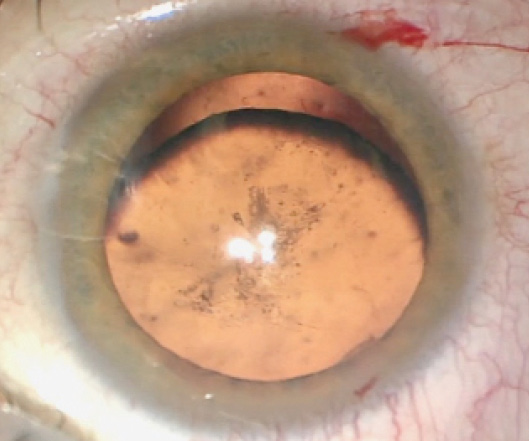
The patient’s visual acuity at the time of referral measures 20/200 OD, and there is no improvement with pinhole. Visual acuity measures 20/20 OS. His right eye is enophthalmic with a full range of movement, and an afferent pupillary defect (APD) is evident that was noted by the neuro-ophthalmologist. A slit-lamp examination of the right eye shows a quiet conjunctiva and sclera. The cornea is clear. Vitreous is visible in the anterior chamber. Iridodonesis is present, as is inferior subluxation of the lens. Posterior subcapsular changes in the lens are also evident (Figure 1). Findings from a slit-lamp examination and a dilated retinal examination of the patient’s left eye are normal.
(Courtesy of Brandon D. Ayres, MD)
Figure 1. A clinical examination of the patient’s right eye showed inferior subluxation of the lens as well as posterior subcapsular cataract formation.
How would you manage the cataract?

ASHVIN AGARWAL, MS
For cases such as this one, I feel it is important to bear in mind two rules:
No. 1: Always approach the vitreous from posterior to the iris plane.
No. 2: When dealing with vitreous in a phaco probe, never yank the instrument for fear of causing a retinal detachment.
I would have on hand the following devices: iris hooks, capsular hooks, capsular tension rings (CTRs), an anterior vitrector set up with a trocar, and a trocar anterior chamber (AC) maintainer. After fashioning the corneal incisions and carefully performing the capsulorrhexis, especially in the area of loose zonules, I would place a trocar AC maintainer and connect an infusion line. To stabilize the bag, I would make a partial-thickness scleral flap at the site of maximum subluxation, and I would create a sclerotomy under the flap with a 22-gauge needle that entered a space behind the iris and in front of the crystalline lens. I would then perform an anterior vitrectomy via the sclerotomy, thereby pulling down the vitreous.
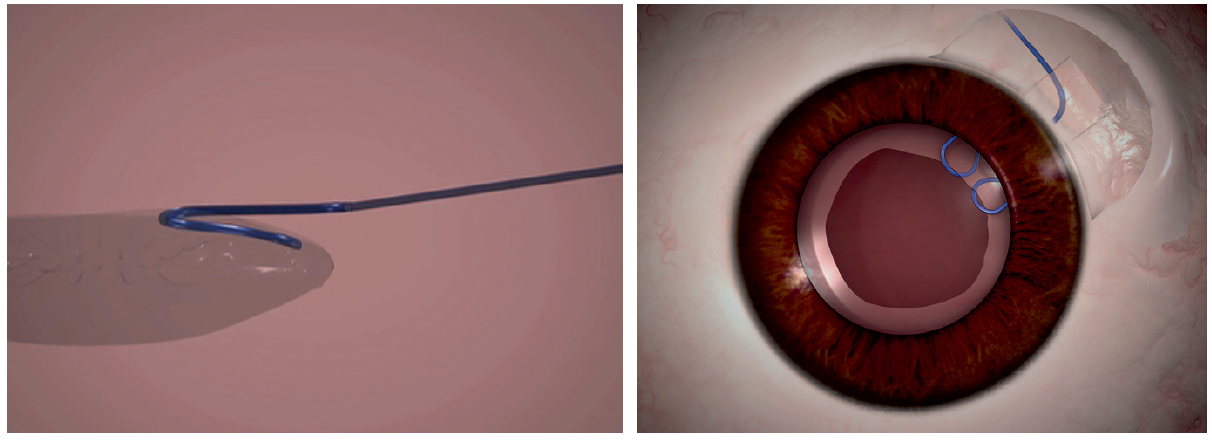
I would introduce a paperclip hook through the main incision (Figure 2). After tucking the proximal part of the clip into the anterior rim of the capsulorrhexis, I would externalize the distal end through the sclerotomy and tuck it into a Scharioth tunnel at the base of the flap, thereby ensuring stability and centration of the capsular bag. Next, with capsular hooks and a CTR in place, I would perform phacoemulsification and then remove the hooks. I would seal the flap with fibrin glue.
(Courtesy of Ashvin Agarwal, MS)
Figure 2. A paperclip capsule stabilizer hooks around the capsulorrhexis to support the capsular bag (left). The hook engages the capsulorrhexis at two points, giving a broad area of contact and a round capsulorrhexis rim (right).

MARJAN FARID, MD
Evidence of optic nerve trauma and a positive APD mean that the refractive goal should be to restore lens and media clarity. I would not consider a multifocal IOL for this patient because the optic nerve has been compromised. Moreover, I would be prepared to encounter total zonular loss and a complete loss of the capsule. Scleral fixation of a primary IOL by tunnel glue or the Yamane technique may prove necessary.
My initial approach would be a thorough AC vitrectomy to minimize the risk of retinal traction. I would then place a heavy cohesive OVD in the AC to prevent further vitreous prolapse. Capsular dye would help me to create a capsulorrhexis centered on the lens, not the pupil. If there were enough zonular support and only segmental loss, I would place capsular tension hooks in that segment to pull the entire lens capsule complex into a central position before starting phacoemulsification. Once the capsule and lens were well centered, I would perform gentle hydrodissection and lens removal using low-vacuum phacoemulsification.
Because the patient is young, I do not expect dense nuclear manipulation to be necessary. I would use quick-chop techniques in order to stay centered and minimize stress on the remaining zonules. After successfully removing the lens, I would proceed with thorough cortical cleanup, followed by placement of a three-piece monofocal IOL in the capsular bag. The spring-like haptics of a three-piece IOL would provide some capsular support, and they will offer versatility if the lens needs to be sutured in the future. Because of the segmental zonular loss in this eye, I would use an Ahmed Capsular Tension Segment (CTS; Morcher) to center and fixate the IOL-capsular bag complex to the sclera.
A femtosecond laser can be helpful for performing a capsulotomy centered on the traumatically displaced pupil. Additionally, presoftening facilitates nuclear removal, with a subsequently minimal need for phaco manipulation.

MAGDA RAU, MD
I do not believe the visual acuity of 20/200 correlates with the inferior subluxation of and posterior subcapsular changes in the lens. The eye is enophthalmic, perhaps owing to shrinkage of the orbital fat after the trauma or orbital fracture (eg, blowout fracture). The presence of an APD shows that there are differences between the patient’s two eyes in the afferent pathway because of retinal or optic nerve disease.
Diagnostic testing to exclude fracture of orbita (orbita tomogram) is required prior to surgery. I would also order visual field testing and an OCT scan to rule out an optic neuropathy and maculopathy. During the preoperative consultation, it would be important to emphasize the risks involved in surgery because of the patient’s history of trauma, the zonular and capsular instability, and the lens subluxation. I would make sure that he was aware of the risk of capsular loss and the subsequent necessity of scleral or iris fixation. I would also emphasize that surgery will improve his visual acuity but that the outcome is limited by presumed optic neuropathy or retinal disease.
My preference would be to perform the operation using general anesthesia. Because vitreous is visible in the AC, a vitrectomy is necessary. I would favor a pars plana vitrectomy over an anterior bimanual vitrectomy out of concern that the latter would increase subluxation of the lens. If the position of the lens after the vitrectomy remained unchanged, then I would instill an OVD, and I would perform the capsulorrhexis using a curved 25-gauge cystotome irrigation cannula with a 0.5-mm port so that I could add an OVD as needed. I would perform the capsulorrhexis cautiously, while watching for further zonular loss and movement of the lens. If I could execute an even, stable capsulorrhexis, I would place capsular hooks and then carefully proceed with gentle hydrodissection and phacoemulsification.
I would expect the lens nucleus to be soft, removable mainly with irrigation and aspiration and nearly no phaco energy. I would suture the capsule with one or two CTSs in addition to passing a suture through sclerotomies located 2 mm posterior to the limbus. Because the presence of a CTS will make cortical removal more difficult, I would combine hydrodissection with viscodissection, irrigation, and aspiration.
If zonular loss were significant and lens subluxation increased during phacoemulsification, I would convert to extracapsular cataract extraction (ECCE), enlarge the incision to 6 to 8 mm, and express the nucleus with balanced salt solution or Healon (Johnson & Johnson Vision). If subluxation became so severe that the lens began to descend, however, I would convert to intracapsular cataract extraction and remove the entire lens with the assistance of an OVD. After completing ECCE, I would inject triamcinolone acetonide and perform further vitrectomy if necessary.
I would proceed with transscleral fixation or iris suturing of a three-piece IOL. Because vision in the patient’s right eye decreased immediately after the accident, and owing to possible retinal disease or optic neuropathy, I would not recommend a multifocal IOL for this patient. I would avoid an ACIOL as well because of his age and the potential for endothelial cell loss. Retro pupillary fixation of an Artisan IOL (Ophtec) or Verisyse lens (Johnson & Johnson Vision) could be considered.

KAROLINNE MAIA ROCHA, MD, PhD
I am concerned that the patient lost vision soon after the injury and that an APD is evident on examination. Together, these findings indicate that he probably had a traumatic optic neuropathy after the accident, but he states that his vision worsened progressively over the past year. It probably got worse because he developed a posterior subcapsular cataract in the setting of lens subluxation.
In terms of managing the patient’s expectations, I would like to determine his visual potential and discuss it with him. A simple step in the clinic would be to use the potential acuity meter. A more sophisticated option would be visual evoked potential testing. Obtaining the records from the neuro-ophthalmologist from a year ago would be helpful in determining the patient’s baseline visual acuity.
A posterior subcapsular cataract can cause significant visual symptoms, especially halos and glare at night, so I definitely think this patient would benefit from surgery.
I would plan to perform laser cataract surgery with a paracentesis and a 2.2-mm cataract incision. The capsulotomy is a limiting factor in cases of traumatic cataract with zonular dehiscence. Because the subluxated lens is decentered and has poor zonular support, it could be difficult to center the capsulotomy on the dislocated capsular bag. In cases like this one, I have found the femtosecond laser useful for creating the anterior capsulotomy, because I can center it on the scanned capsule instead of on the pupil. I would plan a capsulotomy with a diameter of 5 mm using scanned-capsule centration with a femtosecond laser.
Because of the vitreous present in the AC, I would perform an anterior vitrectomy and instill a dispersive OVD. I would use MST Capsule Retractors (MicroSurgical Technology) to center the lens and mechanically stabilize the capsular bag. Then, I would perform slow-motion phacoemulsification and hydrodissection in an effort to preserve the capsular bag. For fixation, I would suture an Ahmed CTS on the sclera, across the area of subluxation. Next, I would implant a one-piece, hydrophobic acrylic IOL in the bag.
I would instill triamcinolone acetonide (Triesence; Alcon). If I observed any residual vitreous, I would complete the anterior vitrectomy. Otherwise, I would instill acetylcholine (Miochol-E; Bausch + Lomb) to constrict the pupil, which vitreous can make irregular.
I would expect this patient to do very well.

WHAT I DID: BRANDON D. AYRES, MD
As in any complex case I undertake, I held a long and comprehensive discussion of the risks and benefits of surgery with the patient. I explained the increased complexity of his case and the uncertainty of his final visual outcome, owing to his history of a traumatic optic neuropathy. After carefully considering his options, the patient decided to proceed with cataract surgery.
The first challenge was creating the continuous curvilinear capsulorrhexis (CCC). The mobility of the lens made maintaining centration of the CCC difficult. After executing the first 180º, I placed capsule retractors, which allowed centration and fixation of the lens. The additional support provided by the capsule retractors permitted me to complete the remainder of the CCC without difficulty. I was careful not to overtension the capsule retractors, which could have allowed a tear-out of the CCC (Figure 3).
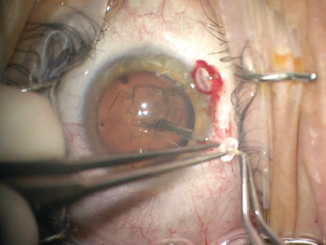
Figure 3. Capsular support hooks added stability to the lens and allowed completion of the capsulorrhexis.
After creating the CCC, I performed hydrodissection and hydrodelineation in my usual fashion. I removed nuclear material with as little rotation of the nucleus as possible so as to minimize trauma to the zonules. I was concerned about a progressive loss of support during the case, especially during cortical cleanup. I used bimanual irrigation and aspiration to minimize trauma and facilitate the removal of cortical lens fibers. After lens removal was complete, I placed a CTR to recruit support from the remaining intact zonular fibers. Then, I implanted a one-piece acrylic IOL in the capsular bag (Figure 4).
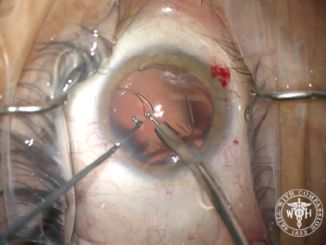
Figure 4. Placement of a capsular tension ring after lens removal. In this case, the capsular support hooks were removed prior to placement of the ring.
Next, I performed a vitrectomy to remove vitreous that had prolapsed from the prior trauma. I initially performed the vitrectomy through two paracenteses and used triamcinolone acetonide to help me visualize the vitreous strands. It became difficult to keep the vitreous posterior, so I transitioned to a pars plana vitrectomy through a 23-gauge valved trochar. The change to a posterior incision gave me much better control of the vitreous, and I was able to clear the surgical incisions. My attention then turned to centering the IOL (Figure 5).
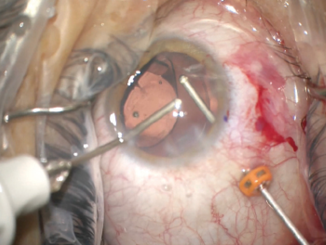
Figure 5. A pars plana approach to anterior vitrectomy. In this case, the flow of fluid from anterior to posterior facilitated the removal of vitreous and prevented its incarceration into the corneal wounds.
I placed an Ahmed CTS to support and center the IOL. Using a 1-mm sharp blade, I created a superior peritomy and two sclerotomies. I secured the CTS to the sclera with a Gore-Tex suture (W.L. Gore & Associates; off-label usage). I used a slipknot to add tension to the suture until the IOL appeared well centered in the eye. I then carefully buried the knot in the sclerotomy (Figure 6). With an 8-0 Vicryl suture (Ethicon), I closed the sclerotomy and peritomy.
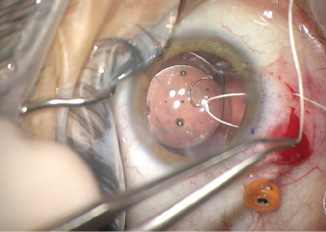
(Figures 3–6 courtesy of Brandon D. Ayres, MD)
Figure 6. Off-label use of a Gore-Tex suture to fixate a capsular tension segment, which was then used to center the IOL. Care was taken to bury the knot of the suture to prevent erosion.
At the conclusion of the case, all OVD was removed from the AC using the vitrector. I checked the corneal incisions to ensure a watertight closure. I instilled carbachol to induce miosis and ensure no vitreous strands remained incarcerated in the corneal wounds.
The patient tolerated the surgical procedure well. On postoperative day 1, he had regained a UCVA of 20/50 and expressed excitement about his visual recovery. Although the postoperative course was uncomplicated, his UCVA had decreased to 20/80 by month 1, but it could be corrected with a minor myopic correction to 20/50. The patient’s examination and visual acuity have remained stable over the past 9 months.
1. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124(8):1136-1142.


