IOL optic capture using the posterior capsule can be an effective strategy to ensure stable IOL fixation in some cases and to prevent phimosis or secondary opacification. Traditionally, the optic is captured by the anterior capsulotomy, but it can also be captured by a posterior continuous capsulotomy. The haptics can be in the sulcus, in the bag, or behind the posterior capsule in the anterior vitreous.
Gimbel first described posterior optic capture in 1990.1 Gimbel and DeBroff subsequently described the various configurations of optic capture,2 some of which are summarized in Table 1. This article discusses a recent variation of posterior capsular capture described by Lisa Brothers Arbisser, MD, that is a modification of Gimbel and DeBroff’s previous work.3

TRADITIONAL OPTIC CAPTURE
Traditional optic capture places the haptics of a three-piece IOL in the sulcus, with the optic posterior to and captured by the continuous anterior capsulotomy. Initially, the entire IOL is placed into the sulcus, and then only the optic is prolapsed posteriorly and captured by a centered continuous capsulotomy (Figure 1).

Figure 1. Traditional optic capture.
This technique is very useful in the setting of a posterior capsular tear. The captured optic of the IOL seals off the tear so that the vitreous is sequestered behind the optic. The IOL is stable and tends to remain centered. The haptics in the sulcus seem to provide immediate support to the IOL in eyes with weak zonules, especially when this technique is aided by the placement of a capsular tension ring (CTR). The capture of the capsule also helps to lessen capsular phimosis, which keeps the lens stable in the long term.4
Pavlina Kemp, MD, and I showed that a common three-piece IOL (MA50, Alcon) placed in the sulcus with no optic capture was at risk for decentration, especially in long eyes.5 The effective position of the optic is about the same with traditional optic capture as when the entire IOL is placed in the bag, so no adjustment of IOL power is required.6 The technique requires a three-piece IOL and is not suitable for use with the large square haptics of a one-piece acrylic IOL.7
OPTIC CAPTURE WITH POSTERIOR CAPSULOTOMY
Gimbel and DeBroff described the use of the posterior capsule to capture the optic.8 The posterior capsule can be used for optic capture when the anterior capsular opening either is too large to capture the optic or is not continuous. In such cases, the surgeon will typically gently place the entire IOL in the bag when the posterior capsule is intact. However, if the posterior capsule is also damaged, it may be possible to extend a posterior capsular tear into a continuous and centered opening to allow capture of the optic.

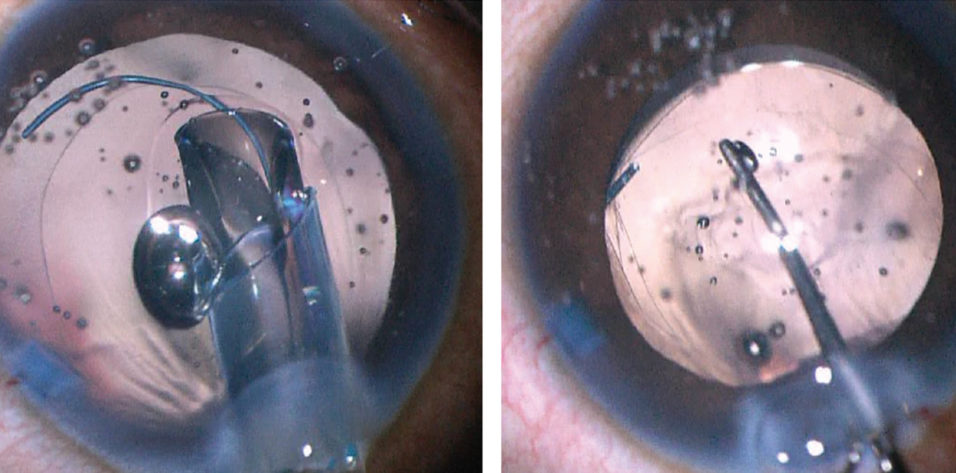
Figure 2. A 27-gauge needle is used to start the posterior capsulorhexis (left). A cohesive OVD is injected behind the posterior capsule to displace the anterior face of the vitreous (right).
Crafting a posterior capsulotomy can be difficult. The first step is to stabilize the anterior chamber and capsular bag with an OVD. Next, a 27-gauge needle or cystotome on a cohesive OVD syringe is used to incise the posterior capsule and then, with OVD injection, to displace the anterior face of the vitreous back without disrupting it (Figure 2). This starts the posterior capsulorhexis, and the OVD helps to keep the capsular tear folding anteriorly toward the surgeon. The posterior tear often uses a shearing type of motion, and microforceps can be useful to control the tear (Figure 3).

Figure 3. Use of a shearing force with microforceps helps to control the size of the capsulotomy in an elastic capsule (left). The primary posterior capsulotomy (middle) should match the size and shape of the anterior capsulotomy (right); both should be about 1 mm smaller than the optic diameter.
An interesting application of posterior optic capture is to prevent posterior capsular opacification (PCO). Gimbel and DeBroff first proposed the posterior capture of the optic with anterior vitrectomy as a technique to prevent PCO in children.8 Vasavada and colleagues recently reported a series of pediatric patients with posterior capsular optic capture who were observed for a year with no significant PCO.9 This finding was confirmed by Zhou and Zhou in a large review of cases by meta-analysis.10 Menapace described an amazing series of 1,000 adult patients with primary posterior capsulotomy and posterior optic capture with rare complications and essentially no PCO formation.11
HYALOID-SPARING DOUBLE CAPTURE
At a recent meeting, Arbisser described a modification of Gimbel and DeBroff’s technique, which she calls “hyaloid-sparing double capture” (HSDC).3 In this variation of capsular capture, after a continuous anterior capsulotomy is created, a CTR can be placed into the capsular bag. Then a planned posterior capsulotomy is performed. After the posterior capsular tear is initiated, a cohesive OVD is used to separate the anterior hyaloid from the capsule, sparing the hyaloid and avoiding the need for anterior vitrectomy.
The anterior and posterior capsulotomies are made about 1 mm smaller than the planned optic size to allow capture of the optic. A three-piece IOL is placed with its haptics in the sulcus and its optic prolapsed all the way back so that it is captured by both the anterior and posterior capsules.
The HSDC technique thus takes advantage of the stable configuration of the traditional optic capture—haptics in the sulcus and phimosis prevention—and the PCO prevention of posterior capture, and it does not require anterior vitrectomy.
INDICATIONS AND RESULTS
HSDC is indicated in patients who are unable to sit for laser capsulotomy (eg, young patients and those with nystagmus or cognitive impairment) or who have a dense posterior capsular opacity and need immediate visual rehabilitation. The bicapsular capture creates a very stable IOL, which is especially useful in patients with zonulopathy.
Table 2 lists details of the 12 cases that my colleagues and I have done at the University of Iowa over the past year using this technique, including several in which we did not complete bicapsular capture but were able to use either traditional optic capture or solely posterior capsular capture.

TECHNIQUE IN DETAIL
The first step of the HSDC technique is to create an anterior capsulotomy about 1 mm smaller than the diameter of the planned IOL. If the anterior capsular opening is too large, then the optic can be captured only by the posterior capsule. If the anterior capsular opening is too small, it can be extended after nucleofractis is performed. After nucleofractis, a CTR can be placed. The CTR makes the posterior capsulotomy easier and helps to lessen phimosis and enhance centration.
The most awkward and unfamiliar next step is to purposely tear the posterior capsule. I prefer to use a 27-gauge needle loaded onto a cohesive OVD syringe (Figure 2, left). Just after needle entry, I inject a cohesive OVD to displace the anterior vitreous face posteriorly and to bring the posterior capsule anterior (Figure 2, right). Using an OVD to bow the posterior capsule forward makes the posterior capsulorhexis more natural and similar to an anterior capsulotomy.
Because many of the cases in our series involved children unable to sit for laser capsulotomy, the posterior capsule was often elastic. Use of shearing forces with microforceps (Figure 3, left) helps to control the size of the elastic capsulotomy. The primary posterior capsulotomy (Figure 3, middle) should match the size of the anterior capsulotomy (Figure 3, right); both should be about 1 mm smaller than the optic diameter.

Figure 4. The IOL is first placed into the sulcus (left). A Kuglen hook is used to prolapse the optic behind both the anterior and posterior capsules (right).
The IOL is first placed into the sulcus (Figure 4, left). The leading haptic of the three-piece IOL is placed just below the iris and guided into the sulcus. The trailing haptic is also placed into the sulcus with a hook or forceps. The IOL is spun with a hook to ensure that both haptics are in the sulcus. The optic is then pushed posteriorly so that the optic is posterior to both the anterior and posterior capsules (Figure 4, right).

Figure 5. The IOL is captured by both the anterior and posterior capsules.
Both the anterior and posterior capsules will often take on an oval shape as they are stretched in the axis of the haptic insertion (Figure 5). If the capsulotomy is not perfectly centered, one can often center the IOL by rotating the axis of the haptics to a symmetric portion of the capsulotomy. If the posterior capsule is too small (Figure 6) or not continuous to allow posterior capture, the optic can simply be captured by the anterior capsule alone in the traditional optic capture configuration.

Figure 6. If the posterior capsule is too small, the optic can be captured by the anterior capsule alone.
SUMMARY
Capture of the IOL optic by either the anterior or posterior capsule can be very useful. Traditional optic capture with the haptics in the sulcus and the optic in the bag is a stable configuration for long-term IOL fixation. Optic capture can also lessen capsular phimosis. Capture of the IOL in the posterior capsulotomy can help to prevent PCO formation, which is especially important in patients who cannot sit for a laser procedure.
The bicapsular optic capture described by Gimbel and DeBroff helps to prevent PCO. Arbisser has now developed the modification described herein using cohesive OVD, which spares the anterior hyaloid. HSDC takes advantage of optic capture with haptics positioned in the sulcus for lens stability, while, at the same time, capture by the posterior capsule lessens PCO formation, a feature that is particularly advantageous in difficult pediatric cases.
1. Gimbel HV. Posterior capsule tears using phacoemulsification: causes, prevention and management. European Journal of Implant and Refractive Surgery. 1990;2:63-69.
2. Gimbel HV, DeBroff BM. Intraocular lens optic capture. J Cataract Refract Surg. 2004;30(1):200-206.
3. Arbisser L. Bicapsular optic capture for prevention of PCO and phimosis. Paper presented at: Iowa Eye Annual Meeting; June 2016; Iowa City, IA.
4. Chang DF. Intraocular lens implantation with abnormal zonules. In: Chang DF, ed. Phaco Chop and Advanced Phaco Techniques. Thorofare, NJ: Slack; 2013:269-270.
5. Kemp PS, Oetting TA. Stability and safety of MA50 intraocular lens placed in the sulcus. Eye (Lond). 2015;29(11):1438-1441.
6. Millar ER, Allen D, Steel DH. Effect of anterior capsulorhexis optic capture of a sulcus-fixated intraocular lens on refractive outcomes. J Cataract Refract Surg. 2013;39(6):841-844.
7. Chang DF, Masket S, Miller KM, et al; ASCRS Cataract Clinical Committee. Complications of sulcus placement of single-piece acrylic intraocular lenses: recommendations for backup IOL implantation following posterior capsule rupture. J Cataract Refract Surg. 2009;35(8):1445-1458.
8. Gimbel HV, DeBroff BM. Posterior capsulorhexis with optic capture: maintaining a clear visual axis after pediatric cataract surgery. J Cataract Refract Surg. 1994;20(6):658-664.
9. Vasavada AR, Vasavada V, Shah SK, et al. Postoperative outcomes of intraocular lens implantation in the bag versus posterior optic capture in pediatric cataract surgery. J Cataract Refract Surg. 2017;43(9):1177-1183.
10. Zhou HW, Zhou F. A meta-analysis on the clinical efficacy and safety of optic capture in pediatric cataract surgery. Int J Ophthalmol. 2016;9(4):590-596.
11. Menapace R. Posterior capsulorhexis combined with optic buttonholing: an alternative to standard in-the-bag implantation of sharp-edged intraocular lenses? A critical analysis of 1000 consecutive cases. Graefes Arch Clin Exp Ophthalmol. 2008;246(6):787-801.
12. Oetting T. Optic capture. In: Agarwal A, ed. More Phaco Nightmares. Thorofare, NJ; Slack; 2018:235.