The main lacrimal gland, residing in the lacrimal fossa of the frontal bone, is believed to be the primary contributor to the aqueous layer of the tear film. In contemporary eye care, substantial attention is given to the main lacrimal gland and its innervation and mechanisms to increase output of the gland in the context of treating dry eye disease (DED). But, what about the accessory lacrimal glands of Krause and glands of Wolfring? This article examines the function and role of these lesser-known lacrimal glands in maintaining ocular health and how they are affected in DED.
KRAUSE AND WOLFRING: THE HIDDEN PLAYERS
There are 20 glands of Krause in the superior conjunctival fornix and 10 glands within the inferior fornix. The glands of Krause are located under the eyelid, where the upper and lower conjunctiva meets. The glands of Wolfring are located within the nonmarginal border of both the superior and inferior tarsal plates (Figure 1).
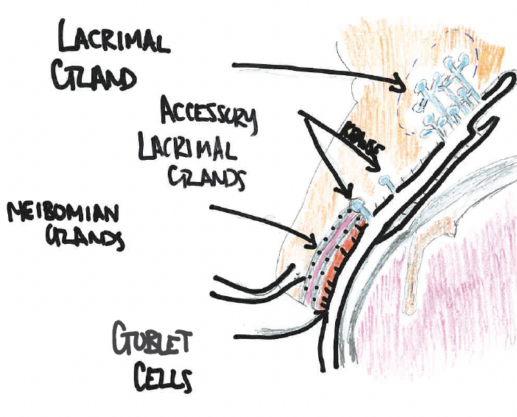
Figure 1. Anatomic landmarks of glands involved in the tear surface.
Histologically, the glands of Krause and Wolfring are similar to the main lacrimal gland. These lacrimal glands account for only 10% of the total lacrimal secretory mass, but their role in ocular surface homeostasis cannot be overlooked. To understand these accessory lacrimal glands, we must first think about the most basic functional unit of the lacrimal glands.

Figure 2. Example of an exocrine gland with acinus and duct. Lacrimal glands, including main and accessory glands, are exocrine glands.
It is hypothesized that these accessory lacrimal glands of Krause and Wolfring are responsible for basal tear secretion, whereas the main lacrimal gland is responsible for reflex tearing.1 In a small study, the lacrimal gland of squirrel monkeys was removed unilaterally.2 Testing of the ocular surface, including Schirmer testing, slit-lamp examination with fluorescein staining, and analysis of tear proteins at weeks 1 and 20 postprocedure were performed. Notably, with removal of the lacrimal gland, there was an 80% decrease in basal tears and a 90% decrease in reflex tears at week 1. However, by week 20, this decrease was noted to be only approximately 30% compared with the control arm. Moreover, there were no differences in corneal fluorescein staining in the majority of the monkeys, and no histologic changes were noted. This study suggests that the accessory lacrimal glands are adequate to maintain ocular surface homeostasis and prevent clinical signs of DED.2
Pathology. Pathology of these glands can be relatively rare. A dacropys is a fluid-filled cyst arising from the lacrimal ductule and can be found in any lacrimal gland tissue. Cases of Wolfring dacropys have been reported as a cause of acquired mechanical ptosis in children. These present as painless, well-circumscribed lesions noted on physical examination and can be confirmed on ultrasound or computed tomography.3<
Other pathologies that can affect the accessory lacrimal glands include chronic inflammation and periductular fibrosis associated with aging and disease (ie, DED).4 In other postmortem studies, there was noted to be a significant increase in this ductular fibrosis and atrophy—more so in post-menopausal women than in men.5 It has even been noted that the palpebral conjunctiva and conjunctival fornix, especially the superior conjunctival fornix, may be vital to both the main and accessory lacrimal gland output. As the lumens of the glands empty into the palpebral conjunctiva and superior conjunctival fornix, any damage or pathology in this anatomic region can result in a significant decrease in tear output to the ocular surface.6 One classic example is that of Stevens-Johnson syndrome, in which a robust inflammatory reaction results in cicatricial damage of the palpebral conjunctiva and fornix.7 Ultimately, patients have severe ocular surface disease compounded with decreased tear output from scarring within the lumens of the lacrimal gland, the glands of Krause, and the glands of Wolfring. Another invading entity damaging the palpebral conjunctiva and fornix is biofilm.
BIOFILM INVASION: A HIDDEN THREAT
Unraveling the mystery of biofilm invasion introduces a new dimension to ocular surface health. Ocular surface biofilm is a network of microorganisms that proliferate from the periocular skin and lashes to the lid margin and up into the palpebral conjunctiva and fornix.8 Due to the anatomic location of the fornix, the biofilm is seldomly disrupted, hence harboring inflammation. As the bacterial load and matrix invade the ductules of the glands of Krause and Wolfring and the excretory ductules of the main lacrimal gland, they inhibit outflow and result in an imbalance of ocular homeostasis, ultimately contributing to dry eye syndrome. Moreover, Demodex infestation of the lashes has been implicated in a dysregulation in the biofilm of the conjunctival fornix.9
THE ROLE OF ACCESSORY LACRIMAL GLANDS: NOT TO BE OVERLOOKED
The accessory lacrimal glands of Krause and Wolfring play an integral role in tear production and ocular surface balance. Their anatomic location in the palpebral conjunctiva and superior conjunctival fornix underscores their importance for ocular surface health.
The biofilm invasion of the palpebral conjunctiva and fornix is often ignored and underrecognized, which, unfortunately perpetuates a symptomatic patient’s disease. Although physicians are apt to treat ocular surface biofilms of the lashes and lid margin, including blepharoexfoliation and meibomian gland expression, we have ignored the biofilm of this anatomic area.
Moreover, treatments that may eradicate Demodex still leave the biofilm of the fornix and palpebral conjunctiva untouched. The Rinsada irrigating eyelid retractor (Rinsada) is a novel in-office device that uses high-pressure irrigation targeted toward the palpebral conjunctiva and fornix. (High-pressure irrigation has been used in other fields of medicine to remove biofilms.8) Rinsada can be performed as a stand-alone procedure for the ocular surface or in combination with other biofilm removal procedures, such as BlephEx (BlephEx), Zocular Eyelid System Treatment (ZEST; Zocular), low-level light therapy, and intense pulsed light therapy.
Armed with the knowledge provided in this article, eye care practitioners should be better able to ensure the ocular surface health of their patients.
1. Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. 1966;62(1):47-60.
2. Maitchouk DY, Beuerman RW, Ohta T, Stern M, Varnell RJ. Tear production after unilateral removal of the main lacrimal gland in squirrel monkeys. Arch Ophthalmol. 2000;118(2):246-252.
3. Khoury NJ, Haddad MC, Tawil AN, Ma’luf RN. Ductal cysts of the accessory lacrimal glands: CT findings. Am J Neuroradiology. 1999; 20(6):1140-1142.
4. Roen JL, Stasior OG, Jakobiec FA. Aging changes in the human lacrimal gland: role of the ducts. CLAO J. 1985;11(3):237-242.
5. Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland: statistical analysis with special reference to aging. Ophthalmology. 1995;102(4):678-686.
6. Conrady CD, Joos ZP, Patel BC. Review: the lacrimal gland and its role in dry eye. J Ophthalmol. 2016;2016:7542929.
7. Rynerson JM, Perry HD. DEBS – a unification theory for dry eye and blepharitis. Clin Ophthalmol. 2016;10:2455-2467.
8. Rhee MK, Yeu E, Barnett M, et al. Demodex blepharitis: a comprehensive review of the disease, current management, and emerging therapies. Eye Contact Lens. 2023;49(8):311-318.
9. Rmaile A, Carugo D, Capretto L, et al. Removal of interproximal dental biofilms by high-velocity water microdrops. J Dent Res. 2014;93:68-73.



