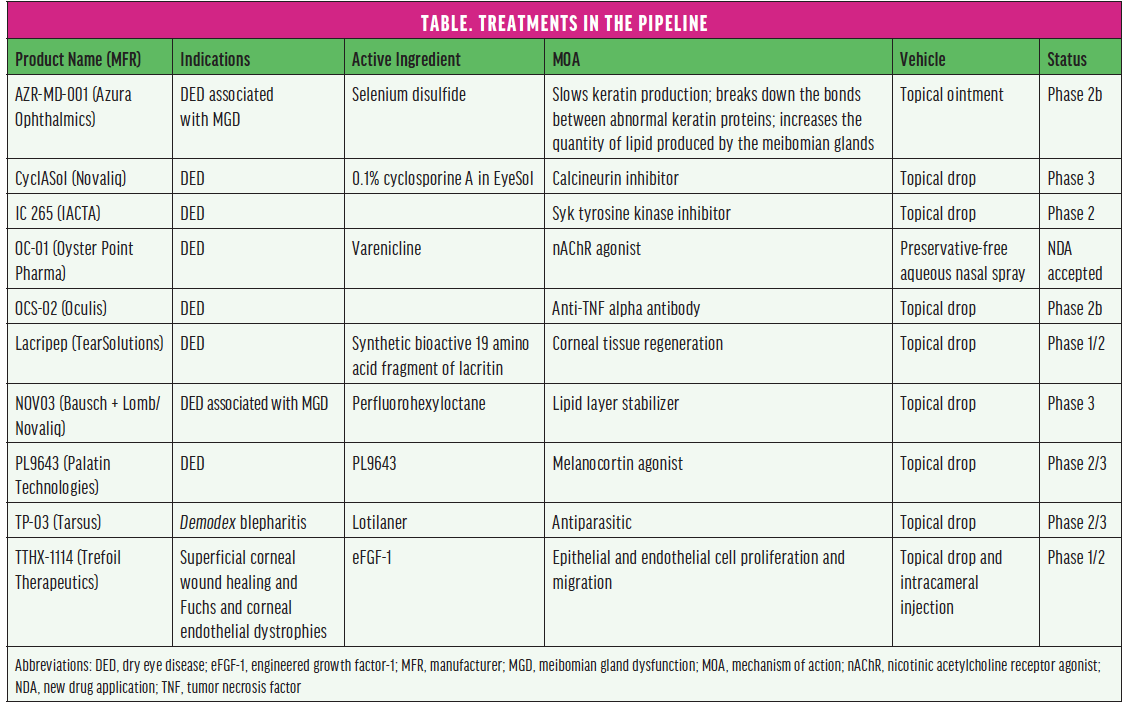
From the foundation of modern approaches to treating ocular surface disease (OSD) to products in the pipeline (see the Table), this article reviews some currently available therapeutic approaches and diagnostic and treatment devices and looks ahead to what’s to come. (Editor’s note: The treatments and devices featured here are not an exhaustive list of products in this sector of eye care.)
CYCLOSPORINE A and a Novel FORMULATION

Pierre-Jean Pisella, MD, PhD
CsA 0.1%
Cyclosporine 0.1% (CsA; Ikervis, Santen Pharmaceutical), in my routine practice, is a second-line prescription for patients with dry eye disease (DED) in combination with tear film substitutes. In my practice, evaporative DED is predominant rather than meibomian gland dysfunction (MGD).
I prescribe Ikervis when a patient presents with a uni- or bilateral superficial punctate keratitis score greater than 1 on the Oxford scale associated with functional (visual) signs and/or classic pain symptoms on clinical examination at two successive visits. Sometimes, predominant symptoms without clinical signs are also an indication.
Evaluation of symptoms and clinical signs is done at 6 months, and, if significant improvement is achieved, CsA 0.1% is continued with controls twice a year.
Tear film substitutes are also continued for all patients, and the difficult, still unresolved question remains: “When do we stop using Ikervis?” This is never an easy question to answer—for the physician or the patient.

Kenneth A. Beckman, MD
CsA 0.05%
Cyclosporine (CsA) 0.05% (Restasis, Allergan) was approved by the FDA almost 20 years ago, forever changing approaches to OSD treatment. Before its emergence, dry eye disease (DED) therapy was limited to palliative treatments such as artificial tears and punctal plugs. When Restasis was approved, ophthalmologists began to focus on DED as an inflammatory disease and determined that treating the inflammation was critical to stopping and even reversing ocular surface damage. Other antiinflammatory dry eye medications have been developed over the 20 years since the approval of Restasis, and numerous treatment algorithms have been developed. Additionally, our understanding of the inflammatory processes of DED continues to grow—as does general public awareness of DED. In recent years, the management of patients with DED has been one of the most significant aspects of practice for many eye care professionals. There is no doubt that Restasis was a major catalyst for the rapid evolution in awareness, knowledge, and treatment of OSD.

Barry A. Schechter, MD
CsA 0.09%
CsA 0.09% (Cequa, Sun Pharma) is an aqueous nanomicellar formulation that was approved by the FDA in 2018 to increase tear production in patients with DED. Because the active ingredient, CsA, is hydrophobic, many prior formulations have been limited in delivering CsA to the inflamed ocular tissues. Nanomicelles, globular structures, have a hydrophobic core that surrounds the CsA molecules and a hydrophilic exterior that solubilizes CsA into the aqueous layer of the tear film to be released to the ocular surface. This attribute, along with the small size (range, 10–80 nm; average, 22 nm) of the nanomicelles, allowed up to a threefold increase in the distribution to the ocular surface tissues than prior available formulations demonstrated in animal studies.1 This is in addition to minimal systemic absorption. Studies have shown that the increased bioavailability of the active drug in Cequa may lead to more rapid resolution of DED-related ocular surface changes. In my experience, it also leads to more satisfied patients. I have used this medication for many of my pre- and postsurgical patients, especially those with OSD and those who choose a premium IOL. It has also been suitable for long-term use in patients who have undergone corneal transplant surgery.
1. Agarwal P, Craig JP, Rupenthal ID. Formulation considerations for the management of dry eye disease. Pharmaceutics. 2021;13(2):207.

Eric D. Donnenfeld, MD
Lifitegrast
Lifitegrast (Xiidra, Novartis) is a small-molecule integrin antagonist that acts as an intercellular adhesion molecule 1 decoy. By blocking the interaction of intercellular adhesion molecule 1 with lymphocyte functional antigen 1, lifitegrast inhibits T-cell adhesion, migration, and activation and subsequent cytokine release. These multiple touch points in the inhibition of dry eye–associated inflammation help account for the rapidity of action of lifitegrast in significantly improving both the signs and symptoms of DED as soon as 2 weeks after the initiation of therapy and is well documented in the FDA trials.1
I currently use Xiidra as a first-line therapy in the management of aqueous-deficient DED. I find it particularly helpful for rapidly rehabilitating the ocular surface in patients contemplating ocular surgery and, specifically, cataract surgery. In the clinical trials, a common adverse event was dysgeusia.1 Interestingly, this has not been a significant concern among my patients, whereas they occasionally report burning and irritation. I have found combined immunomodulation with a topical corticosteroid and lifitegrast to dramatically reduce this adverse event and more rapidly improve DED signs and symptoms.
1. Holland EG, Whitely WO, Sall K, et al. Lifitegrast clinical efficacy for treatment of signs and symptoms of dry eye disease across three randomized controlled trials. Curr Med Res Opin. 2016;32(10):1759-1765.
CORTICOSTEROID DROPS

Alice T. Epitropoulos, MD, FACS
Fluorometholone acetate ophthalmic suspension 0.1%
Fluorometholone acetate ophthalmic suspension 0.1% (Flarex, Eyevance Pharmaceuticals) is approved by the FDA for the treatment of inflammatory diseases of the ocular surface and anterior segment, the most common of which is DED resulting from inflammation of the ocular surface, tears, and lacrimal glands. The objective in treating DED is to break the inflammatory cycle. The short-term administration of Flarex can calm inflammation and often helps patients feel and see better.
It is important to note that not all fluorometholone molecules are the same. The derivative in various preparations differs, which can affect tissue penetration. Flarex contains an acetate group that improves lipophilicity, allowing greater penetration to target tissues and resulting in a more potent effect compared with fluorometholone alcohol. In a pivotal FDA trial, Flarex achieved a statistically significant improvement in the speed with which signs and symptoms resolved compared with fluorometholone alcohol (P = .05).1 Despite its greater potency, fluorometholone acetate ophthalmic suspension 0.1% is a hydrogenated ester and is considered to be a safer steroid with a better side effect profile than fluorometholone alcohol.
To my mind, fluorometholone acetate ophthalmic suspension 0.1% provides the best of both worlds: the potency of a strong steroid and the side effect profile of a mild steroid.
1. Leibowitz HM, Hyndiuk RA, Lindsey C, Rosenthal AL. Fluorometholone acetate: clinical evaluation in the treatment of external ocular inflammation. Ann Ophthalmol. 1984;16(12):1110-1115.

Preeya K. Gupta, MD
KPI-121 0.25%
KPI-121 0.25% (Eysuvis, Kala Pharmaceuticals) is a novel formulation of loteprednol etabonate that has an excellent safety and efficacy profile, as shown in robust clinical trials.1-3 In my clinical practice, using topical steroids is critical to managing patients with OSD. Many patients who are on long-term topical immunomodulators or other therapies for DED experience acute flare-ups of their disease, and I have been using Eysuvis to treat these acute episodes because it is not only the first FDA-approved product for the short-term treatment of DED but also one that has demonstrated an excellent safety profile.
I am finding that, when I treat acute flares, patients do better for longer in terms of remission of symptoms. I believe, with greater awareness of flare disease, we are finding more patients who experience flares but don’t identify themselves as having DED. These patients also require treatment, and this novel molecule is effective in managing the signs and symptoms of acute DED flare disease.
1. Safety and efficacy of KPI-121 in subjects with dry eye disease (STRIDE 1). clinicaltrials.gov identifier: NCT02813265. Updated February 3, 2021. Accessed April 28, 2021. https://clinicaltrials.gov/ct2/show/NCT02813265?term=KPI-121+0.25%25&draw=2&rank=3
2. Safety and efficacy of KPI-121 compared to placebo in subjects with dry eye disease (STRIDE 2). clinicaltrials.gov identifier: NCT02819284. Updated January 5, 2021. Accessed April 28, 2021. https://clinicaltrials.gov/ct2/show/NCT02819284?term=KPI-121+0.25%25&draw=2&rank=2
3. Safety and efficacy of KPI-121 in subjects with DED (STRIDE 3). clinicaltrials.gov identifier: NCT03616899. Updated April 2, 2021. Accessed April 28, 2021. https://clinicaltrials.gov/ct2/show/NCT03616899?term=KPI-121+0.25%25&draw=2&rank=5
DIAGNOSTIC DEVICES

Brandon D. Ayres, MD
InflammaDry
My referral practice focuses on anterior segment and corneal pathology. The InflammaDry (Quidel) point-of-care test is used in three different scenarios in our clinic. First, it is one of our core point-of-care tests used to diagnose OSD and to manage patients newly diagnosed with OSD. All new patients are evaluated for the presence of matrix metalloproteinase 9 in the tear film. A positive result indicates that excessive inflammatory mediators are present in the tear film and that the patient may benefit from topical antiinflammatory medications. A patient already on immunomodulatory medications who has a positive test may need additional or more aggressive therapy.
Second, InflammaDry is used to assess the effect of treatment. Three to four months after beginning a new treatment, patients are retested to see if the level of inflammation in the tear film has decreased.
The third way InflammaDry is used is in screening patients for OSD before anterior segment surgery. Any patient who answers positively on an intake questionnaire screening for DED receives this test. Patients with a positive result are evaluated more closely for clinically significant OSD and treated, if necessary, before surgery.

Neda Nikpoor, MD
LipiScan
At our practice, every patient who comes in for a refractive or cataract surgery consultation gets a LipiScan (Johnson & Johnson Vision) to evaluate for meibomian gland atrophy, tortuosity, and areas of dropout. We use this information as well as information from tear film analysis (HD Analyzer, Visiometrics) and a slit-lamp examination to assess the ocular surface. We also look at the epithelial thickness map and OPD-3 (Nidek), iTrace (Tracey Technologies), or Pentacam (Oculus Optikgeräte) quality scans to assess the level of OSD before laying eyes on the patient.
I find the LipiScan is a great visual tool that allows us to show the patient what anatomical changes are present that explain their symptoms. It is also useful to have an objective marker to show to asymptomatic patients who may be prone to developing OSD symptoms after surgery. I find this helps them understand the importance of pretreating meibomian gland dysfunction (MGD) before any eye surgery.
Anatomy and function do not always correlate, so I personally digitally express the meibomian glands of every new surgical consult, even if their LipiScan is normal. If there is significant disease on LipiScan or if the patient has symptoms, I always prefer to pretreat the patient’s OSD and repeat measurements before eye surgery, especially if they are interested in laser vision correction or an advanced technology IOL.

Christopher E. Starr, MD
TearLab Osmolarity System
I consider tear osmolarity (TearLab Osmolarity System, TearLab) to be my desert island DED test. If I could have only one, this would be it. The two most widely cited and adopted consensuses and evidence-based algorithms—ASCRS Preoperative OSD Algorithm and TFOS DEWS II Diagnostic Algorithm—both recommended tear osmolarity as a first-line DED test. Although a basket of objective data (eg, matrix metalloproteinase 9, noninvasive tear breakup time, meibography, lipid layer thickness) is always better, tear osmolarity remains the most fundamental of the DED point-of-care tests.

Marguerite B. McDonald, MD, FACS
Keratograph 5M
The Keratograph 5M (Oculus) is a true workhorse. I think it is indispensable to any anterior segment practice in ophthalmology and optometry, particularly those that have or are developing a dry eye center of excellence (for more on this subject, see “A Stepwise Guide to Building an OSD Service in Your Practice,” pg 27). The Keratograph 5M uses a color camera, four illumination systems, and three magnification settings to perform an array of tests for DED assessment. It quickly evaluates tear film quality and quantity, tear film dynamics, noninvasive keratometric breakup time, bulbar hyperemia, fluorescein imaging, lissamine green imaging, lid and lash imaging, and meibomian gland morphology (meibography). It also has a Placido-based topographer that is useful for detecting corneal disease, contact lens fitting, and more. Meibography is incredibly important to my patients; it can motivate them to stay compliant with at-home care and to consider undergoing in-office procedures such as microblepharoexfoliation and thermal pulsation.
The Keratograph 5M automatically generates a Crystal Tear Report, which allows me to rapidly develop an ocular surface evaluation and treatment protocol in the following three phases:
It captures pictures, videos, and measurements acquired with the device during the capture phase.
It assesses the imaging, measurements, and patient symptoms during the assessment phase.
It produces a treatment plan from a preselected list of treatments that is specific to the condition and its severity. The treatment recommendations are created during the assessment phase. The doctor has complete control over the treatment plan. The final report is printed and given to the patient.
TREATMENT DEVICES

Richard A. Adler, MD
Intense Pulsed Light
Multifactorial diseases require multifactorial treatments, and the use of intense pulsed light (IPL) to complement traditional OSD treatments is an example. IPL offers an alternative to pharmacology to control the inflammatory basis of OSD, positioning IPL to be one of the only device options available to address both evaporative and aqueous-deficient forms of DED. By treating the root, upstream inflammatory etiologies of OSD, IPL targets its origins, not only its downstream consequences. IPL helps to ensure a durable, lasting improvement in ocular surface function.
Advances in light technology have resulted in the safe, comfortable treatment of both the upper and lower lids, with easy-to-use interfaces and preset parameters that promote an effective and efficient treatment. For patients not responsive to traditional therapies, unable to use drops compliantly, or discouraged by the side effects of topical medications, IPL offers a drug-free, drop-free, insurance-free solution to OSD.

Neil J. Friedman, MD
iTear100
Neuromodulation is an established therapeutic strategy for a variety of diseases. For DED, neurostimulation of the lacrimal nerve, initially with intranasal microelectric pulses (TrueTear, Allergan) and more recently with external mechanical oscillation (iTear100, Olympic Ophthalmics), is safe and effective. The iTear100, which has clearance from the FDA and is available to patients by prescription, is a pocket-sized device that is applied to the external nasal region to stimulate the trigeminal nerve and activate the parasympathetic nerve pathway controlling tear film homeostasis. This device produces both immediate and sustained responses, suggesting increased basal lacrimal secretion. It is easy to use and well tolerated by patients, and its use improves the signs and symptoms of DED.1 The iTear100 is a welcome addition to our dry eye treatment armamentarium. I find it useful for all stages of DED.
1. Ji MH, Moshfeghi DM, Periman L, et al. Novel extranasal tear stimulation: pivotal study results. Trans Vis Sci Technol. 2020;9(12):23.

Elizabeth Yeu, MD
LipiFlow
MGD is a leading cause and exacerbator of OSD. LipiFlow (Johnson & Johnson Vision) has allowed me to effectively help patients in preparation for cataract surgery and to augment the efficacy and/or mitigate the need for other DED therapies. In my experience, the patients who experience the most symptomatic improvement from treatment have functional MGD with minimal to no structural meibomian gland damage.

Mitchell A. Jackson, MD
TearScience LipiView II Ocular Surface Interferometer
The TearScience LipiView II Ocular Surface Interferometer (Johnson & Johnson Vision) offers real-time visualization of the lipid layer to evaluate the dynamic response of lipids to blinking, patented noise-canceling technology to measure the submicron thickness of the lipid layer, video and analysis of blink dynamics, and high-definition imaging with Dynamic Meibomian Imaging. This technology aids in the proper decision-making process for meibomian gland treatment in a customized fashion.
Meibography has historically been challenging due to limitations in meibomian gland visualization with traditional surface illumination techniques. Their structure, however, is critical in assessing the severity of DED.1,2 One benefit of the LipiView II is that it uses dynamic illumination surface lighting rather than standard white or infrared illumination. Imaging of the meibomian glands using standard white light illumination is obstructed by blood vessels and infrared illumination. Even though this technology is capable of seeing past the blood vessels, it can produce glare due to the convex surface of the eyelid. Because dynamic illumination surface lighting combines these two approaches and originates from multiple light sources, reflection is minimized, and meibomian gland imaging capability is optimized.
Another advantage of this diagnostic device is the ability to quantify the percentage of meibomian gland dropout. In my experience, for patients with greater than 50% meibomian gland dropout, other thermal pulsation treatments will have minimal if any benefit to the patient. I tell my patients that, if they have glands, they can have treatment. Showing them their actual meibomian imaging in the examination lane can make their decision to proceed with treatment that much easier.
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283.
2. Wolffsohn JS, Arita R, Chalmers R. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-574.

Ivan Mac, MD, MBA
Systane iLux MGD Thermal Pulsation System
More than 86% of DED cases are thought to have an MGD component.1 Reduced meibum can decrease the quality of the tear film and lead to evaporative DED, so treatment strategies that don’t address this key feature are often incomplete and ineffective.
The Systane iLux MGD Thermal Pulsation System (Alcon) is one of the tools that we use in the clinic to help manage OSD. We use it early in the disease course in an effort to avoid meibomian gland atrophy in the long term. Treatment typically lasts 10 minutes and is usually repeated annually to maintain the effect. Acceptance is high among my patients because they understand that this treatment targets the primary underlying disease mechanism of their OSD.
1. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017; 28(Suppl 1):3-47.

William B. Trattler, MD
TearCare System
MGD can be a challenging condition to treat, and the symptoms related to evaporative DED can be quite significant. Although traditional therapies can at times be helpful, many patients benefit from a procedure that liquefies thickened meibum and unclogs the glands, which can result in a significant improvement in the quality of the gland secretions. Thermal pulsation with LipiFlow has been the gold standard in this area, and it has an excellent track record. Symptomatic patients can experience a significant improvement in their symptoms following treatment with LipiFlow.
In the past few years, the TearCare System (Sight Sciences) has also been available to significantly improve symptoms. TearCare, along with LipiFlow and iLux, are easy on both the eye care provider and the patient, and the benefits of improved tear film quality can be profound. In my experience, patients often experience improvement that lasts from 6 months to many years, making both of these technologies important parts of the management of dry eye patients.
THE PIPELINE

Laura M. Periman, MD
AZR-MD-001
Despite the incorporation of multiple in-office treatments for MGD, unmet needs and clinical challenges remain. One of the six interrelated mechanisms of MGD is obstruction, and hyperkeratinization is an unaddressed component. This physical challenge to evacuation of dysfunctional meibum reminds me of hair and cooking grease that are clogging a drain and blocking flow. It’s not enough simply to melt the grease; you also have to break up the hair. Keratin disulfide bonds are broken only by high heat (well outside of human tissue tolerability limits) or chemical reduction (selenium sulfide). There are currently no approved pharmacological treatments for terminal duct obstruction due to hyperkeratinization associated with MGD. Fortunately, a novel strategy for addressing the hyperkeratinization component of MGD is in phase 2b clinical trials, and the significant improvements from AZR-MD-001 0.5% and 1.0% (Azura Ophthalmics) in meibomian gland secretion and meibum quality are highly encouraging.1
1. Methods to enhance AZR-MD-001 for meibomian gland dysfunction (MGD). ClinicalTrials.gov Identifier: NCT04314362. Updated January 11, 2021. Accessed April 28, 2021. https://clinicaltrials.gov/ct2/show/NCT04314362?term=AZR-MD-001&draw=2&rank=3

John A. Hovanesian, MD
CsA 0.05% and 0.1% dissolved in EyeSol
CyclASol (CsA 0.05% and 0.1% dissolved in EyeSol, Novaliq) is a unique and powerful way to deliver CsA to the eye. Because the vehicle is a water-free, high–molecular weight hydrocarbon, CsA is highly soluble in it, unlike water. The drop size of this drug is about one-fifth the volume of a normal 50-µL eye drop, so it is almost imperceptible when placed in the eye. Because it is an oil-like organic material, it coats the eye with a very thin layer that increases the surface area for absorption of the drug. Because the vehicle is water-free, it is unfriendly to bacterial or fungal overgrowth, so no preservatives are necessary. In the age of novel drug delivery, the formulation of new products is as important as pharmacology. With CyclASol, an old and trusted drug may achieve even higher success because of the way it is delivered.

John P. Berdahl, MD
IC 265
IC 265 (IACTA) holds promise as a fast-acting, well-tolerated antiinflammatory for DED, which would be an important step forward in addressing the large remaining unmet needs in the dry eye space.1 Another exciting aspect of this molecule is its potential to reduce redness, similar to what was seen in phase 2 clinical trials investigating IC 265 for allergy (data on file with IACTA). If that translates into redness reduction and fast-acting symptomatic improvement for patients with DED, it will help them tremendously. Results from the phase 2 clinical trial may be available by early 2022.
1. Harmon D. Market Scope dry eye analyst report. 2014.

Christophe Baudouin, MD, PhD, FARVO
OCS-02
There is a true unmet medical need for the development of drugs with new mechanisms of action to treat the inflammation involved in the pathogenesis of DED. OCS-02 (Oculis), which is being developed as a topical antitumor necrosis factor alpha, may fill that gap. Compared with other product candidates in the DED pipeline, OCS-02, given its antiinflammatory and antinecrosis benefits, could play a role in the treatment of the underlying causes of the disease. Of particular interest is the potential of a genetic biomarker identified in a phase 2a study to help personalize treatment. Further investigation is warranted, but the possibility of identifying the right treatment for the right patient is appealing considering the multifactorial and complex nature of DED. Early clinical results for OCS-02 showed a favorable safety and tolerability profile, whereas currently approved treatments are not always well tolerated, which can decrease patients’ adherence to treatment (data on file with Oculis).

Marc G. Odrich, MD
Lacripep
Lacripep (TearSolutions) is a topical tear-promoting, ocular-health-restorative and tear film–stabilizing peptide of the parent tear protein lacritin. This National Eye Institute–supported discovery from the University of Virginia has been validated and extended by multiple national and international laboratories over the past 20+ years. Natural tear levels of both Lacripep and lacritin are insufficient in all forms of DED. Recently, Lacripep was the subject of a large double-masked, randomized, placebo-controlled, multicenter phase 2 clinical trial that enrolled patients with primary Sjögren Syndrome DED. After only 2 weeks of treatment, Lacripep had minimized both inferior corneal staining and patient complaints of burning and stinging (P = .005 and .006, respectively).1 This novel replacement therapy can restore ocular surface homeostasis naturally. A phase 3 trial in the general dry eye population is planned for the near future.
1. Lacripep in Subjects With Dry Eye Associated With Primary Sjögren’s Syndrome. ClinicalTrials.gov Identifier: NCT03226444. Updated January 22, 2020. Accessed April 28, 2021. https://clinicaltrials.gov/ct2/show/NCT03226444?term=Lacripep&draw=2&rank=1

Joseph Tauber, MD
NOV03
The antievaporative properties of NOV03 (Bausch + Lomb and Novaliq) make it an ideal replacement therapy to retain a functional tear film for patients with no functioning meibomian glands. Its other properties, lubrication, and possible oil-liberating actions make it a great choice for patients with less advanced forms of either evaporative or aqueous-deficient DED. Studies so far show the product is well tolerated and effective at improving both the signs and symptoms of DED. This preservative-free unique molecule will likely be a game-changer in dry eye therapy.1
1. Tauber J, Wirta DL, Sall K, Majmudar PA, Willen D, Krösser S; SEECASE study group. A randomized clinical study (SEECASE) to assess efficacy, safety, and tolerability of NOV03 for treatment of dry eye disease. Cornea. Publish online December 22, 2020. doi:10.1097/ICO.0000000000002622

Kenneth Kenyon, MD
PL9643
Palatin Technologies is exploring a novel strategy in the ever-broadening area of dry eye therapy with its topical preparation of PL9643, a melanocortin receptor agonist with potential ocular antiinflammatory activity. In a recently completed phase 2 FDA clinical trial, adults with DED were randomly assigned to receive either PL9643 or vehicle three times daily for 12 weeks. When analyzed by subgroup, adults with moderate to severe DED demonstrated a statistically significant improvement in inferior corneal fluorescein staining and ocular discomfort (using the Ora Calibra Drop Comfort Scale). Improvements were notable by week 2 and sustained through week 12. Moreover, PL9643 was well tolerated, and only a few mild adverse events occurred.1
As a cornea and external disease specialist, I am excited to witness the development of another potential antiinflammatory therapeutic approach to DED therapy with the hallmarks of demonstrable early objective and subjective efficacy, patient comfort, and objective ocular surface improvement. Additional studies will potentially reinforce the development of PL9643 as the first in the melanocortin agonist class to treat DED.
1. Efficacy and safety of PL9643 ophthalmic solution in subjects with dry eye. ClinicalTrials.gov Identifier: NCT04268069. Updated November 13, 2020. Accessed April 28, 2021. https://clinicaltrials.gov/ct2/show/NCT04268069

Arthur B. Cummings, MB ChB, FCS(SA), MMed(Ophth), FRCS(Edin)
TearClear
The TearClear product line hits the mark in three crucial areas for me:
1. No benzalkonium chloride (BAK) comes into contact with the eye, so ocular surface health and patient compliance are promoted.
2. BAK in the bottle maintains sterility while not in use.
3. One bottle is used per month instead of 30+ single unit plastic vials, which dramatically reduces plastic waste.
The product range is almost limitless because the TearClear platform is designed to deliver potentially any BAK-preserved eye drop as a pristine, preservative-free dose. Look out for an expanding range of eye drops, starting with glaucoma medications.

Ehsan Sadri, MD
TP-03
TP-03 (Tarsus) is a novel therapeutic that, once approved by the FDA, will be the first treatment for Demodex blepharitis. TP-03 will meet a substantial need for millions of patients. It is designed to paralyze and eradicate mites and other parasites through the inhibition of parasite-specific gamma-aminobutyric acid-chloride ion channels.
In the phase 2 clinical trials, in which I participated, TP-03 was well tolerated by patients, and there were no significant adverse events that led to treatment discontinuation. Pivotal phase 2b and 3 clinical trials are currently underway. I’m excited about our early results with this therapeutic and believe it could lead to other potential uses, such as for dermatology.

Deepinder K. Dhaliwal, MD, LAc
TTHX1114
TTHX1114 (Trefoil Therapeutics) can be used to treat diseases affecting the corneal epithelium and endothelium. This engineered fibroblast growth factor compound mimics the natural regenerative biologic processes associated with cell proliferation and migration, hastening corneal epithelial regeneration and wound healing.
Corneal ulcers can be caused by a variety of diseases and injuries such as ulcerative keratitis, abrasions, and chemical or burn damage. These represent future opportunities for topical TTHX1114 to meet important clinical needs. TTHX1114 has shown potential in a chemical injury model in studies supported by a grant from the US Department of Defense.1
Intracameral TTHX1114 is also being developed for the treatment of Fuchs dystrophy and other corneal endothelial dysfunctions such as pseudophakic bullous keratopathy. A phase 2 safety trial has been completed with no dose-limiting toxicity or adverse events reported. A second phase 2 study is currently enrolling for treatment in conjunction with Descemet stripping only to demonstrate accelerated recovery of vision (Figure).
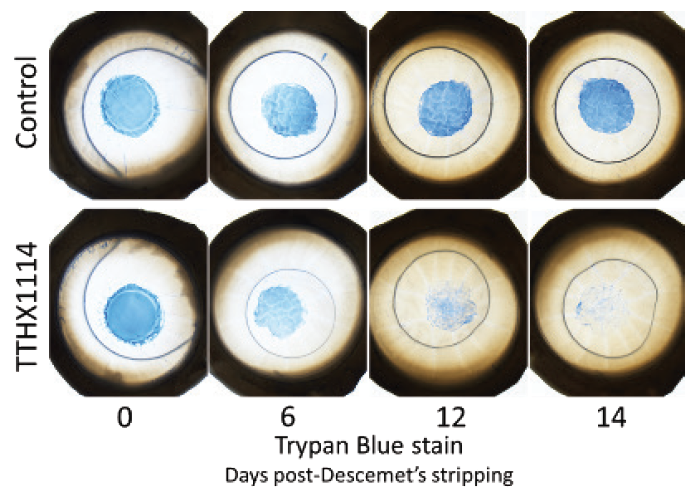
Figure. The effect of TTHX114 on human donor corneal tissue following Descemet stripping only. Note the rapid endothelial cell proliferation and migration in the corneas treated with TTHX1114.
1. A phase 1/ phase 2 study of TTHX1114(NM141) (INTREPID). US National Library of Medicine. March 23, 2021. Accessed April 22, 2021. https://clinicaltrials.gov/ct2/show/NCT04520321?term=TTHX1114&draw=2&rank=3

Vance Thompson, MD, FACS
Varenicline
With more than 1,500 different proteins, including growth factors and antibodies, as well as numerous classes of lipids and mucins, the eye’s tear film is complex. As a result, there is no tear better than the patient’s own tear. That is why I get so excited about neurostimulation. It increases all three layers of a patient’s tear film, including the aqueous, mucin, and meibum. This natural tear film production can reestablish tear film homeostasis. OC-01 nasal spray (Varenicline, Oyster Point Pharma) is a nicotinic acetylcholine receptor agonist that stimulates the trigeminal parasympathetic pathway in the nasal cavity. Clinical trials involving my practice have found an improvement in both DED signs and symptoms in patients treated with this preservative-free nasal spray. The ONSET-2 phase 3 study of OC-01 evaluated doses of 0.6 mg/mL and 1.2 mg/mL.1 Both of these treatment groups showed a statistically significant improvement in the Schirmer score of 10 mm or more at week 4 versus vehicle and the patients receiving 1.2 mg/mL showed a significant improvement in symptoms as early as week 2 and at week 4. Another benefit with neurostimulation of tear production is that it avoids the ocular surface toxicity associated with certain dry eye treatments.
1. Oyster Point Pharma announces positive results in ONSET-2 phase 3 trial of OC-01 nasal spray for the treatment of the signs and symptoms of dry eye disease [press release]. Oyster Point Pharma. May 11, 2020. Accessed April 21, 2021. https://investors.oysterpointrx.com/news-releases/news-release-details/oyster-point-pharma-announces-positive-results-onset-2-phase-3


