The short answer to the question posed in the headline is obviously: “It varies.” Prescribers of spectacle lenses know from subjective refractions that changes of 0.25 D of cylinder (DC) are noticeable to most patients with normal eyes. The exact cylinder refraction is routinely included in the spectacle prescription, leaving zero residual cylinder, because there is usually no additional cost to the patient to do so. In the absence of significant spherical error, the decision of whether the cylinder must be corrected is based on the individual's symptoms and his or her in-office impression of the improvement in vision that can be achieved with the refraction in place.
For other refractive correction methods, however, such as contact lenses and cataract surgery, there is a significant cost difference between spherical and spherocylindrical correction (excluding relaxing incisions or compensating incision placement), so the decision to proceed with a toric lens—whether IOL or contact lens—must be justified by an expectation of significant visual benefit.
In order to minimize cost and complexity, large contact lens manufacturers usually sell toric lenses with limited steps of power and axis starting at 0.75 DC. The result is that a large number of contact lens wearers are required to function with residual astigmatic errors of up to this amount. Because there does not seem to have been a significant outcry from the contact lens population that this is unacceptable, it may be reasonable to expect that refractive surgery patients can cope with similar residuals. But can the residual astigmatism be even larger? And if residual astigmatism can be avoided or eliminated, should it be?
ASTIGMATISM CORRECTION OPTIONS
Correction of astigmatism at the time of cataract surgery is most commonly achieved through incision placement, creation of peripheral corneal relaxing incisions, toric IOL implantation, or a combination of these;1 the use of toric IOLs is arguably emerging as the most accurate and stable of these techniques.2,3
In one clinical study of 517 astigmatic individuals who were randomly assigned to implantation with either a toric or spherical IOL, the group that received toric IOLs achieved significantly better distance UCVA and spectacle independence. Also, the subgroup of patients who had corneal astigmatism between 0.75 and 1.50 DC and received the toric IOL showed significantly better distance UCVA than the same subgroup in those receiving the spherical IOL.
AT A GLANCE
- Because of the cost to the patient associated with spherocylindrical correction, the decision to proceed with a toric lens—whether IOL or contact lens—must be justified by an expectation of significant visual benefit.
- Data suggest that most astigmatic patients are either receiving alternative surgical treatments for astigmatism or are continuing to be supplied with a best spherical solution.
- The amount of astigmatism that will remain after surgery is difficult to predict because it depends on multiple factors, including the accuracy of biometric data, wound gape or healing effects from limbal relaxing incisions, and toric IOL rotation or displacement.
- Neural adaptation is unlikely to be a long-term consideration in patients with astigmatism, so surgeons should not shy away from its full correction in all patients.

Figure 1. Toric IOL units sold as a percentage of the total IOL market.5
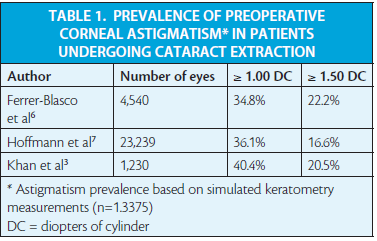
Despite increasing use of toric IOLs, the rate of toric IOL sales (Figure 1) is still much lower than the published prevalence of corneal astigmatism of 1.00 DC or greater, or a more conservative criterion of 1.50 DC or greater (Table 1).3,5-7 These data suggest that most astigmatic patients are either receiving alternative surgical treatments for astigmatism or are continuing to be supplied with a best spherical solution.
Performing corneal incisions on-axis leads to a mean corneal flattening effect of approximately 0.60 D depending on the position and size of the incision, which can substantially reduce the number of patients meeting the criteria for implantation of a toric IOL.8
For individual surgery candidates, the strategy for management of existing astigmatism is based on a complex variety of factors, including reluctance of the patient or public health service to pay the extra cost of a toric IOL, the need to minimize surgical complexity, the refractive status of the fellow eye, and an attempt to provide an extended depth of focus effect.9
EFFECTS OF RESIDUAL ASTIGMATISM
The amount of astigmatism that will remain after surgery is difficult to predict because it depends on multiple factors, including the accuracy of biometric data,10,11 wound gape or healing effects from limbal relaxing incisions,3 and toric IOL rotation or displacement.2,11 This lack of predictability is one of the motivating forces behind the development of adjustable IOL technologies that allow residual sphere and cylinder to be fine-tuned after surgery, potentially reducing the complexity of presurgical calculations and IOL placement.11 (Editor's note: Please see the January 2015 issue of CRST Europe for in-depth coverage of modifiable IOL technologies.) It has also driven the development of intraoperative aberrometry,12,13 which has now developed from static to dynamic assessment.14,15
In laboratory studies, residual astigmatism has a predictable impact on monocular visual acuity.16,17 The blur strength of a pure (sphere-compensated) cylinder error of a given power is equivalent to that of a sphere of half that power.
The visual impact of astigmatism on distance and near tasks can by confounded by the effects of any accompanying spherical defocus.18,19 In a study on the effects of sphere-compensated astigmatism on vision, it was shown that correction of astigmatism as low as 1.00 D significantly improved objective and subjective measures of functional vision in prepresbyopes, both at distance and near.9 In this study, each diopter of sphere-compensated cylinder resulted in a loss of acuity of 0.15 logMAR (about 1.5 lines) for high, medium, and low contrast letters, which is similar to previously published rates.16,17 The study also found that against-the-rule (ATR) cylinder had less impact than the same amount of with-the-rule (WTR) or oblique cylinder on both distance and near visual acuity.
In presbyopes, when simultaneous-vision multifocal refractive correction has been applied, the magnitude of residual astigmatism also affects visual performance. In 2000, Hayashi and coworkers compared the effect of induced myopic astigmatism on monocular visual acuity in patients with monofocal and multifocal IOLs.19 Although distance visual acuity was similar between the monofocal and multifocal IOL groups when no residual astigmatism was present, they found that, with 0.50, 1.00, and 1.50 D of residual astigmatism, the distance acuity was significantly worse with the multifocal IOL. A similar result has been found with more recent multifocal IOL designs with a bench adaptive optic system.20
In clinical studies, residual astigmatism is one of the most significant causes of dissatisfaction after multifocal IOL implantation.21 Residual astigmatism also affects the visual results of other forms of refractive surgery for presbyopia. For example, modeling of the optics of corneal inlays suggests that higher levels of astigmatism should be corrected in order to optimize the depth of focus provided by these devices.22
The use of monovision or partial over-correction of one eye is relatively common in lens-based presbyopia correction. Surprisingly, the combined presence of residual astigmatism with monovision affects binocular visual acuity to a much greater extent than residual astigmatism without monovision.23 This occurs because, if both eyes have optimal spherical correction but residual astigmatism in different axes, the binocular summation of blur allows a blurred astigmatic meridian from one eye to be partly compensated by the same clear meridian in the fellow eye. On the other hand, in a monovision arrangement, the summation process cannot compensate for the blurred meridian due to residual astigmatism because the fellow eye always has spherical defocus present. In monovision, therefore, it is important to correct low amounts of residual astigmatism.

Some of the functional vision impacts of residual astigmatism are reduced over time through adaptation. Adaption to astigmatism seems to occur, at least in relatively young individuals, in a matter of minutes, although the adaptation is orientation-dependent.24,25 The effect of age on ability and rate of adaptation is unclear. It has been suggested that there is a long-term memory effect in adaptation and that there are binocular interactive effects.26 Correcting astigmats of 1.00 D or greater with a toric IOL has been shown to result in better distance visual acuity than correction with a monofocal best sphere, suggesting limited long-term adaptation effects that should be of concern to refractive surgeons.27 However, near acuity was not measured in this investigation, and the effect was not stratified by the level of astigmatism corrected.
CONCLUSION
Clinical evidence suggests that reducing residual astigmatism to less than 0.50 DC will positively affect the outcome of refractive surgery. In presbyopic patients not being considered for a simultaneous-vision multifocal correction, surgeons should be careful not to further reduce an existing low level of ATR astigmatism, as this cylinder may aid the patient's postsurgical spectacle independence.
The age-related change from WTR to ATR astigmatism is, fortuitously, beneficial in that it increases the range of clear focus in the presbyope.28 In monovision correction, any residual astigmatism causes a disproportionate loss of visual acuity. Neural adaptation is unlikely to be a long-term consideration in patients with astigmatism, so surgeons should not shy away from its full correction in all patients. n
1. Amesbury EC, Miller KM. Correction of astigmatism at the time of cataract surgery. Curr Opin Ophthalmol. 2009;20:19-24.
2. Hirnschall N, Gangwani V, Crnej A, et al. Correction of moderate corneal astigmatism during cataract surgery: Toric intraocular lens versus peripheral corneal relaxing incisions. J Cataract Refract Surg. 2014;40:354-361.
3. Khan MI, Muhtaseb M. Prevalence of corneal astigmatism in patients having routine cataract surgery at a teaching hospital in the United Kingdom. J Cataract Refract Surg. 2011;37:1751-1755.
4. Holland E, Lane S, Horn JD, et al. The AcrySof toric intraocular lens in subjects with cataracts and corneal astigmatism. Ophthalmology. 2010;117:2104-2111.
5. Data estimates on file. Abbott Medical Optics, Europe.
6. Ferrer-Blasco T, Montés-Micó R, Peixoto-de-Matos SC, et al. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35:70-75.
7. Hoffmann PC, Hütz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23 239 eyes. J Cataract Refract Surg. 2010;36:1479-1485.
8. Borasio E, Mehta JS, Maurino V. Torque and flattening effects of clear corneal temporal and on-axis incisions for phacoemulsification. J Cataract Refract Surg. 2006;32:2030-2038.
9. Wolffsohn JS, Bhogal G, Shah S. Effect of uncorrected astigmatism on vision. J Cataract Refract Surg. 2011;37:454-460.
10. Koch DD, Ali SF, Weikert MP, et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38:2080-2087.
11. Villegas EA, Alcon E, Rubio E, et al. Refractive accuracy with light-adjustable intraocular lenses. J Cataract Refract Surg. 2014;40:1075-1084.
12. Packer M. Effect of intraoperative aberrometry on the rate of postoperative enhancement: retrospective study. J Cataract Refract Surg. 2010;36:747-755.
13. Chen M. Correlation between ORange (Gen 1, pseudophakic) intraoperative refraction and 1-week postcataract surgery autorefraction. Clin Ophthalmol. 2011;5:197-199.
14. Bhatt UK, Sheppard AL, Shah S, et al. Design and validity of a miniaturized open-field aberrometer. J Cataract Refract Surg. 2012;39:36-40.
15. Kruger RR, Shea W, Zhou Y, et al. Intraoperative, real-time aberrometry during refractive cataract surgery with a sequentially shifting wavefront device. J Refract Surg. 2013;29:630-635.
16. Remon L, Tornel M, Furlan WD. Visual acuity in simple myopic astigmatism: influence of cylinder axis. Optom Vis Sci. 2006;83:311-315.
17. Atchison DA, Mathur A. Visual acuity with astigmatic blur. Optom Vis Sci. 2011;88:E798-E805.
18. Singh A, Pesala V, Garg P, et al. Relation between uncorrected astigmatism and visual acuity in pseudophakia. Optom Vis Sci. 2013;90:378-384.
19. Hayashi K, Hayashi H, Nakao F, et al. Influence of astigmatism on multifocal and monofocal intraocular lenses. Am J Ophthalmol. 2000;130:477-482.
20. Zheleznyak L, Kim MJ, MacRae S, et al. Impact of corneal aberrations on through-focus image quality of presbyopia-correcting intraocular lenses using an adaptive optics bench system. J Cataract Refract Surg. 2012;38:1724-1733.
21. de Vries NE, Webers CAB, Touwslager WRH, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37:859-865.
22. Tabernero J, Artal P. Optical modeling of a corneal inlay in real eyes to increase depth of focus: Optimum centration and residual defocus. J Cataract Refract Surg. 2012;38:270-277.
23. Collins MJ, Goode A, Brown B. Distance visual acuity and monovision. Optom Vis Sci. 1993;70:723-728.
24. Sawides L, Marcos S, Ravikumar S, et al. Adaptation to astigmatic blur. J Vis. 2010;10:22.
25. Ohlendorf A, Tabernero J, Schaeffel F. Neuronal adaptation to simulated and optically-induced astigmatic defocus. Vision Res. 2011;51:529Y34.
26. Yehezkel O, Sagi D, Sterkin A, et al. Learning to adapt: Dynamics of readaptation to geometrical distortions. Vis Res. 2010;50:1550-1558.
27. Sasaki H, Yoshida M, Manabe S, et al. Effects of the toric intraocular lens on correction of pre-existing corneal astigmatism. Jpn J Ophthalmol. 2012;56:445-452.
28. Hayashi K, Masumoto M, Fujino S, et al. Change in corneal astigmatism with aging. Nippon Ganka Gakkai Zasshi. 1993;97:1193-1196.
Gurpreet K. Bhogal-Bhamra, BSc(Hons), MCOptom, PhD
- Specialist Optometrist, Eye Infirmary, New Cross Hospital, Wolverhampton, United Kingdom
- gurpreet.bhogal@nhs.net
- Financial disclosure: None
Michael J. Collins, DipAppSc(Optom), MAppSc, PhD
- Professor of Optometry, Queensland University of Technology, Brisbane, Australia
- m.collins@qut.edu.au
- Financial disclosure: None
Ross Franklin, BAppSc(Optom)
- Senior Research Fellow, Queensland University of Technology, Brisbane, Australia
- r.franklin@qut.edu.au
- Financial disclosure: None
Sunil Shah, MBBS, FRCOphth, FRCS(Ed)
- Midland Eye, Solihull, West Midlands, United Kingdom
- Birmingham & Midland Eye Centre, Birmingham,
United Kingdom
- sunilshah@doctors.net.uk
- Financial disclosure: None
James S. Wolffsohn, BS(Hons), MBA, PhD
- Deputy Dean, School of Life and Health Sciences,
Aston University, Birmingham, United Kingdom
- j.s.w.wolffsohn@aston.ac.uk
- Financial disclosure: None
