The application of ocular biomechanical properties in clinical research, diagnostics, and surgical treatment planning is an emerging area of research that is becoming increasingly relevant to ophthalmic surgery.1 Brillouin optical microscopy is a promising new technology being developed to detect subtle differences in the biomechanical properties of the cornea. Its potential importance stems from recent developments in the understanding of the cornea's role in the measurement of IOP, the impact of the changes induced by keratorefractive surgery on the structural integrity of the cornea, and the introduction of new techniques to alter the biomechanical properties of the cornea such as CXL.
The cornea behaves as a viscoelastic material. The biomechanical properties of this tissue are determined by factors including the organization of collagen fibers, ground substance, and cells. Elastic materials exhibit a constant ratio for all levels of stress or strain, but, for viscoelastic materials, the ratio changes at different levels of stress or strain: The response of the cornea to a force (such as IOP) is dependent not only on the deformation in that moment but also at all previous times.2,3 To predict the results or effects of surgical techniques or to predict the progression of corneal ectasia, the changes in biomechanics induced by intervention or disease must be characterized instantaneously and over time.
MEASUREMENT OF CORNEAL BIOMECHANICS
Current in vivo techniques to measure corneal biomechanical properties require nonphysiologic deformation of the cornea. The Ocular Response Analyzer (Reichert Technologies) delivers a pulse of collimated air to the cornea and monitors the cornea throughout deformation and recovery with an electrooptical system that tracks the corneal reflex with infrared light. The difference between the in- and outward pressure values is reported as corneal hysteresis (CH); a corneal resistance factor (CRF) is calculated using a linear relationship between the two pressures. The Corvis ST (Oculus Optikgeräte) similarly deforms the cornea with a collimated air puff and adds the use of a high-speed Scheimpflug camera to allow dynamic observation of the deformation process. The Corvis ST provides a number of quantitative outputs including deformation amplitude (DA), a measure of the highest displacement of the corneal apex during the highest concavity momentum.4 Both systems aim to characterize the biomechanical properties of the central 3 mm of the cornea, but they do not represent the peripheral structure, which may be particularly relevant in ectatic disorders of the cornea, and they lack the capability to capture regional differences in corneal biomechanical properties.3
Other techniques coupling deformation of the cornea with analysis of high-speed imagery have been proposed, such as swept-source OCT5,6 or supersonic shear wave imaging technology.7,8 As with the ORA and the Corvis ST, these techniques, which are not available commercially for clinical use, rely on nonphysiologic deformation of the cornea.
BRILLOUIN MICROSCOPY IN THE CORNEA
To ideally characterize the biomechanical properties of the cornea, a device capable of measuring regional differences in modulus across the surface of the cornea would be desirable. In order to achieve this goal, it is necessary to obtain measurements without deforming the cornea, as the material properties of the system are affected by stress on the system.
Brillouin optical microscopy has been proposed as a potential solution to the challenge of measuring corneal biomechanics in vivo without altering stress through the analysis of light scatter. Scattering arises from the interaction of photons of incident light with the acoustic phonons in the corneal tissue. In simple terms, a phonon is the unit of vibration of the lattice structure that makes up a material. Photons gain or lose energy from interaction with phonons, and this change (gain or loss) corresponds with a shift in frequency in the Brillouin spectrum of the scattered light. This shift is related to the elastic modulus (M´) of the material, as shown in this equation where ρ = mass density, λ = wavelength, and n = the refractive index.
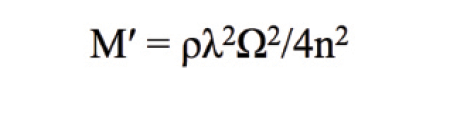
Techniques to calculate the elastic modulus of ocular tissues using Brillouin scatter date back to 1980, when Vaughan and Randall used this technique ex vivo to measure the density and elastic moduli of bovine corneas and the crystalline lenses of cows, rats, birds, fish, and frogs.9 Recently, this technique has been used in laboratory studies to evaluate the effect of CXL on the cornea.1,10,11

Scarcelli et al10 used a benchtop Brillouin microscopy setup to measure the frequency shift in porcine corneas following CXL according to several different protocols, including epithelium-off and transepithelial modalities. The frequency shift in the Brillouin spectrum was used to calculate the longitudinal elastic modulus of the material at various depths. The technique was sensitive enough to detect differences in the amount of stiffening from anterior to posterior cornea and to detect differences between CXL protocols.10
Despite historical understanding of the principles of Brillouin light scattering and potential new applications for this technology, challenges to its implementation as a clinical tool exist. The intensity of scattered Brillouin light is low, and its frequency shifts are small. This necessitates the use of a single-frequency laser, large collection efficiency confocal microscopy optics, and a spectrophotometer with an ultrasensitive detector. These aspects of the design make the measurement system sensitive to temperature, vibration, and alignment. Although these factors are readily controlled in a laboratory environment, their translation into a robust clinical tool is complex. Avedro has taken on this challenge and is currently developing a commercial Brillouin optical microscope, which is attracting attention for its potential as an ocular diagnostic and surgical planning tool.
DIAGNOSTIC, SURGICAL PLANNING TOOLs
Microstructural studies have revealed differences in the collagen lamellar organization in keratoconic corneas compared with normal corneas, with disruption of the orthogonal arrangement over the region of the cone. A reduced number of crosslinks between collagen fibrils in keratoconic corneas contributes to compromise of the lamellar structural integrity and may result in slippage between lamellae (ie, lamellar creep) and progression of the disease. A biomechanical model proposed by Roberts and Dupps hypothesized that keratoconus starts with a regionalized reduction in elasticity that results in lamellar creep, leading to protrusion of the apex of the cone in response to IOP-induced stress and redistribution of stress within the cornea.12 A means of detecting regional variations in corneal biomechanics could be used to validate this theory. If the theory holds true, characterization of regional biomechanics has the potential to provide a means of early diagnosis of keratoconus, opening the possibility of stabilizing the cornea with CXL even before corneal curvature has been affected.
AT A GLANCE
- The application of ocular biomechanical properties in clinical research, diagnostics, and surgical treatment planning is an emerging area of research that is becoming increasingly relevant to ophthalmic surgery.
- Brillouin optical microscopy is a promising technology being developed to detect subtle differences in the biomechanical properties of the cornea.
Currently, the efficacy of clinical CXL procedures is evaluated indirectly; stabilization or decrease in maximum keratometry (Kmax) is the most widely used efficacy endpoint for CXL studies in the scientific literature, with 1.00 D considered the minimum significant change.13 Kmax provides useful clues regarding the progression of keratoconus but does not fully characterize the effect of CXL. As new methods for CXL, such as accelerated protocols and advanced riboflavin formulations, become more widely accepted, it will be useful to quantify in vivo changes in corneal biomechanical properties to evaluate the relative efficacy of these protocols. Brillouin microscopy has the potential to characterize not only changes in the stiffness of the cornea after CXL but also the distribution and magnitude of these changes in three dimensions.10
Studies using Brillouin microscopy to characterize the biomechanical properties of corneas with iatrogenic ectasia have the potential to elucidate the underlying mechanisms of this complication. Measurement of corneal biomechanics may reveal individual predisposition to mechanical instability, leading to better screening to prevent ectasia or to reduce refractive surprises. Additionally, better characterization of biomechanical changes resulting from refractive surgery would be useful to evaluate new refractive surgical procedures aimed at maintaining corneal integrity, such as the ReLEx SMILE procedure.14
Ultimately, preoperative corneal biomechanics measurements could be combined with corneal topography and finite element analysis to create a predictive surgical planning tool for corneal refractive and therapeutic procedures.
BEYOND THE CORNEA
Brillouin microscopy has been used to characterize the crystalline lens and may be the first in vivo diagnostic tool with the capability to differentiate between cortical and lenticular changes in the biomechanical properties of the lens.15-17 In this capacity, it is a potential tool for understanding the progression of presbyopia and the formation of cataracts and to assist in the diagnosis and development of new treatments.
Corneal biomechanical properties influence the measurement of IOP. Both the ORA and the Corvis ST are used to calculate cornea-corrected IOP, which is proposed to be less influenced by corneal properties than traditional applanation tonometry.4 Beyond more accurate measurement of IOP, nondisruptive biomechanical measurement with Brillouin microscopy has the potential to elucidate the pathophysiology of glaucoma and improve clinical monitoring of the disease through biomechanical characterization of the nerve fiber layer, the lamina cribrosa, and the trabecular meshwork.1
Additional applications for biomechanical characterization of the eye may extend to the evaluation of a wide range of ocular features, such as retinochoroidal fluid dynamics and scleral rigidity.
CONCLUSION
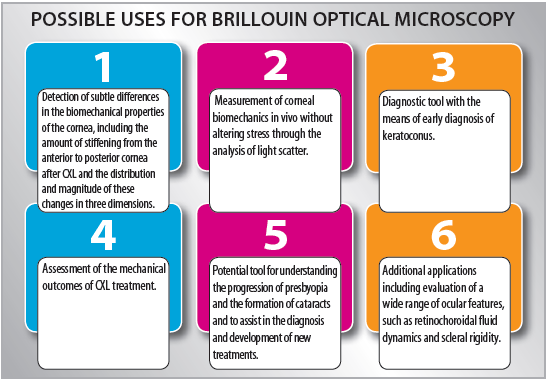
The ability to detect subtle differences in the biomechanical properties of ocular tissues has wide-ranging promise and implications for clinical research, diagnostic tools, and surgical treatment planning. Brillouin optical microscopy is a promising technology with the potential to advance current understanding of regional ocular biomechanical properties through noncontact, real-time in vivo measurement to enable better diagnosis and treatment of disorders affecting or affected by these properties (see Possible Uses for Brillouin Optical Microscopy). n
Dr. Thompson wishes to thank Grace Lytle, OD, and Marc Friedman of Avedro for assistance in the preparation of this article.
1. Girard MJ, Dupps WJ, Baskaran M, et al. Translating ocular biomechanics into clinical practice: current state and future prospects. Curr Eye Res. 2014;1-18.
2. Vellara HR, Patel DV. Biomechanical properties of the keratoconic cornea: a review. Clin Exp Optom. 2015;98(1):31-38.
3. Piñero DP, Alcón N. Corneal biomechanics: a review. Clin Exp Optom. 2014:1-10.
4. Piñero DP, Alcón N. In vivo characterization of corneal biomechanics. J Cataract Refract Surg. 2014;40(6):870-887.
5. Ford MR, Dupps WJ, Rollins AM, Roy AS, Hu Z. Method for optical coherence elastography of the cornea. J Biomed Opt. 2011;16(1):016005.
6. Dorronsoro C, Pascual D, Pérez-Merino P, Kling S, Marcos S. Dynamic OCT measurement of corneal deformation by an air puff in normal and cross-linked corneas. Biomed Opt Express. 2012;3(3):473-487.
7. Tanter M, Touboul D, Gennisson J-L, Bercoff J, Fink M. High-resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE Trans Med Imaging. 2009;28(12):1881-1893.
8. Touboul D, Gennisson J, Nguyen T-M, et al. Supersonic shear wave elastography for the in vivo evaluation of trans-epithelial corneal collagen cross-linking. Invest Ophthalmol Vis Sci. 2014;55(3):1976-1984.
9. Vaughan J, Randall J. Brillouin scattering, density and elastic properties of the lens and cornea of the eye. Nature. 1980;284:489-491.
10. Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013;54(2):1418-1425.
11. Reiss S, Stachs O, Guthoff RF, Stolz H. Advances in Brillouin microscopy of the cornea. Paper presented at: 9th International Congress of Corneal Cross-Linking; December 6-7, 2013; Dublin, Ireland.
12. Roberts CJ, Dupps WJ. Biomechanics of corneal ectasia and biomechanical treatments. J Cataract Refract Surg. 2014;40(6):991-998.
13. Meek KM, Hayes S. Corneal cross-linking - a review. Ophthalmic Physiol Opt. 2013;33:78-93.
14. Mastropasqua L, Calienno R, Lanzini M, et al. Evaluation of corneal biomechanical properties modification after small incision lenticule extraction using Scheimpflug-based noncontact tonometer. Biomed Res Int. 2014;2014:290619.
15. Randall J, Vaughan J. The measurement and interpretation of Brillouin scattering in the lens of the eye. Proc R Soc London Ser B, Biol Sci. 1982;214(1197):449-470.
16. Reiss S, Sperlich K, Hovakimyan M, et al. Ex vivo measurement of postmortem tissue changes in the crystalline lens by Brillouin spectroscopy and confocal reflectance microscopy. IEEE Trans Biomed Eng. 2012;59(8):2348-2354.
17. Bailey ST, Twa MD, Gump JC, Venkiteshwar M, Bullimore M, Sooryakumar R. Light-scattering study of the normal human eye lens: elastic properties and age dependence. IEEE Trans Biomed Eng. 2010;57(12):2910-2917.
Vance Thompson, MD
- Founder, Vance Thompson Vision, Sioux Falls, South Dakota
- vance.thompson@vancethompsonvision.com
- Financial disclosure: Researcher and Consultant (Avedro)


