
Keratoconus is a bilateral progressive corneal ectatic disease inducing visual impairment due to irregular astigmatism or corneal opacity. In its most severe forms, it may require corneal transplantation. CXL is a surgical technique described by Seiler et al in 2003,1 aimed at stabilizing the cornea and halting the progression of keratoconus.
Conventional CXL (C-CXL), although effective at halting progression, is sometimes lacking in providing meaningful improvement in visual acuity. Customized or topography-guided CXL (TG-CXL) has demonstrated the potential to optimize the standard procedure and improve the quality of vision provided by normalizing the corneal curvature.
AT A GLANCE
• Biomechanical analyses of keratoconic corneas have shown that weakening is concentrated within the area of the cone.
• Targeting the CXL procedure to stiffen the area of the keratoconic cone specifically represents one strategy to redistribute biomechanical stresses in the cornea.
• Topography-guided CXL has been shown to induce a flattening effect that is greater than the effect of conventional CXL and also produce a gradient in the biological response to treatment from the area of the cone to the surrounding area.
In C-CXL, the corneal stroma is soaked with a riboflavin solution before exposure to a uniform beam of UV-A radiation. However, biomechanical analyses of keratoconic corneas have shown that weakening is concentrated within the area of the keratoconic cone.2 Targeting the CXL procedure to stiffen this area specifically represents an interesting strategy to redistribute biomechanical stresses in the cornea. Two recent studies, one conducted by Seiler and colleagues3 in Switzerland and the other by our study group in France, led by one of us (FM), underline the great potential of this new technique.4
Both trials were prospective, comparative, nonrandomized clinical studies comparing C-CXL with TG-CXL. Twenty and 30 patients, respectively, were enrolled in the treatment groups in the Swiss and French studies.
TG-CXL PROCEDURE
The details of the TG-CXL procedure as applied in the French study are described below. Minor procedural differences from the procedure utilized by Seiler et al are identified when they appear significant. After administration of topical tetracaine anesthetic, the epithelium was removed mechanically over the region designated for UV-A treatment. Dextran-free riboflavin 0.1% (VibeX Rapid; Avedro) was instilled on the debrided stroma every 2 minutes for 10 minutes. A UV-A irradiation pattern was programmed for each patient using the Mosaic System (Avedro). The pattern consisted of three superimposed concentric circular zones centered on the area with the maximum posterior elevation, as determined by the posterior float parameter on the WaveLight Oculyzer II (Alcon).
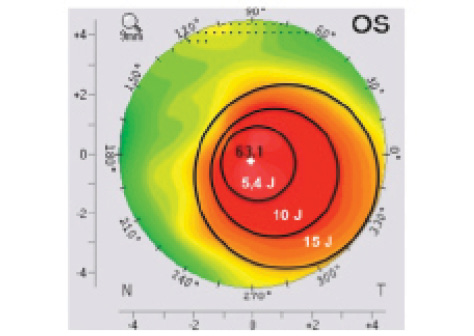
Figure 1. Customized TG-CXL treatment overlaid on a corneal topography map. The maximum UV-A energy (15 J) is focused at the top of the cone, and energy is decreased gradually, from 10 to 5.4 J, toward the periphery of the cone.
The diameter of the inner region encompassed the area of abnormal posterior elevation and the location of maximum keratometry (Kmax). The outer circles were placed to encompass the remaining region of abnormal anterior axial curvature. Transitions between zones occurred at the regions of greatest change in anterior axial corneal curvature. Total energy delivered across the treatment zone ranged from 5.4 J/cm2 in the outermost zone to 15 J/cm2 in the innermost zone (Figure 1). UV-A power was 30 mW/cm2, pulsed at 1-second intervals. Seiler’s team applied lower fluence irradiation, with a maximum total dose of 10 J/cm2 for the innermost treatment zone, with 9 mW/cm2 continuous irradiation. In that study, UV-A treatment profiles for TG-CXL included three concentric circular zones centered on the area with maximum posterior elevation to mimic a graded treatment pattern based on the theoretical model proposed by Dupps et al, which suggests that spatial variation of UV-A intensity results in the greatest flattening effect.3
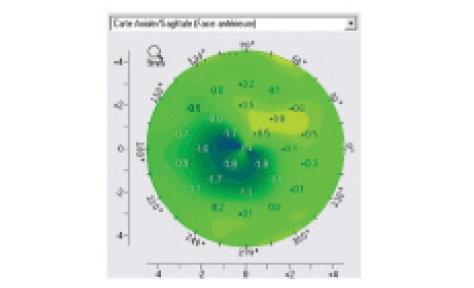
Figure 2. This differential map shows corneal shape change from preoperative to month 12 postoperative.
At the end of the TG-CXL procedures, topical ofloxacin antibiotic (Quinofree; Théa) and a therapeutic contact lens were applied. Postoperative treatment included continuation of the topical antibiotic for 7 days plus the NSAID drop flurbiprofen (Ocufen; Horus Pharma) for 15 days, started at 1 week postoperatively.
RESULTS
Epithelial healing. In both studies, patients were followed for 1 year to evaluate the safety and efficacy of the TG-CXL procedure. In the Swiss study, a significantly shorter time to epithelial healing was observed in the TG-CXL group (2.56 ±0.5 days) than in the C-CXL group (3.19 ±0.73 days).

Kmax. Significant reduction of Kmax was observed in the TG-CXL group in both the French and Swiss studies (-1.07 ±1.70 and -1.07 ±2.00 D, respectively). The reduction in Kmax with TG-CXL was also significantly higher than that in the C-CXL groups in both studies.3,4

Corneal reshaping. To analyze the corneal reshaping effect after TG-CXL (Figure 2), two approaches have been proposed. The Seiler group defined a regularization index corresponding to the maximal steepening plus maximal flattening in the difference map at 1 year versus preoperative. They observed significant differences relative to baseline values after TG-CXL (P=.03). The Malecaze group used two indices derived from Rabinowitz formulas: The superior index (S index) corresponds to the mean K values from five superior points on the 3-mm diameter circle (crossing the 30°, 60°, 90°, 120°, and 150° axes), and the inferior index (I index) corresponds to the mean K values from five inferior points on the same circle (crossing the 210°, 240°, 270°, 300°, and 330° axes). A normalization analysis was performed corresponding to the change in I and S indexes at 1 year versus preoperative values. A significant decrease of I index at 1 year was observed (-0.966 ±1.204; P<.001), with no significant change in S index (0.645 ±1.832; P=.059).

Visual acuity. CDVA improved to 0.2648 ±0.2574 logMAR (P=.104) in the C-CXL eyes, compared with 0.2162 ±0.2495 logMAR (P<.05) in the TG-CXL eyes. Improvement by at least 1 line on the Monoyer scale was seen in 45.6% of C-CXL eyes and 64.5% of TG-CXL eyes. No changes in visual acuity were observed in 24.1% of C-CXL eyes and 12.9% of TG-CXL eyes. Loss of at least 1 line on the Monoyer scale was seen in 30.3% of C-CXL and in 22.6% of TG-CXL eyes.

Safety. Similar ocular side effects were observed in both groups, and these included itching, pain, and blurry vision that quickly improved within the first week upon reepithelization. There were no persistent ocular side effects. Endothelial cell count was stable at all follow-up visits in the TG-CXL group.

Nerve and cell density. Epithelium-off (epi-off) CXL induces significant alterations in corneal stromal components. Nerve and cell density in the cone area decreased immediately after treatment and recovered progressively in both TG-CXL and C-CXL eyes. The effect on nerve and cell density was similar in both the surrounding area and the cone area in C-CXL eyes, but, in TG-CXL eyes, we observed biologic differences between the area of the cone and the surrounding area that did not receive UV-A irradiation. In the TG-CXL group, we noted an asymmetric haze, more pronounced in the cone area than the surrounding side. This haze was similar in appearance to haze observed in the C-CXL group, differing only in its localization to the cone area.

Demarcation line. An indirect clinical outcome of CXL efficacy is the corneal demarcation line observed on OCT (Figure 3). It is best viewed at 1 month, and, at this interval, its depth within the cone area was not statistically significantly different between the TG-CXL and C-CXL groups, indicating comparable efficacy of the TG-CXL technique and the traditional technique.
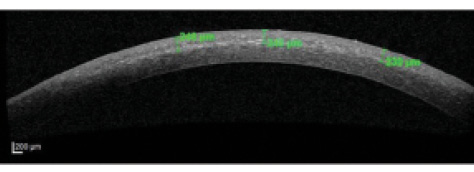
Figure 3. Anterior segment OCT image shows stromal demarcation line (depth indicated in green).
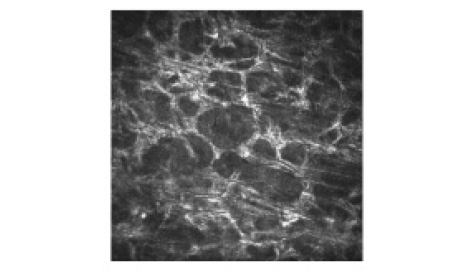
Figure 4. Corneal confocal microscopy image shows activated keratocytes.
Within the surrounding area, a significantly shallower and less pronounced demarcation line was observed than in the treated area (242 ±46 and 182 ±59 µm, respectively, P<.0001), with less significant denervation and cell apoptosis (Figure 4). The cells and nerves recovered more quickly in the surrounding area than in the cone area in TG-CXL eyes. Three complementary considerations could contribute to explaining this biological gradient effect:
Consideration No. 1. It is well known that CXL leads to chemokine release. The corneal stromal architecture is a collagen network that can enable chemokines to diffuse into the untreated corneal area and induce local damage. This paracrine effect could be described as a sponge effect, and it is probably insufficient alone to explain the biological alterations observed.
Consideration No. 2. Riboflavin may have diffused upward in the corneal stroma, and environmental exposure to UV-A irradiation may have occurred, as patients were not advised to wear sunglasses beyond the first few hours after treatment.
Consideration No. 3. Mechanical bias may have had an effect due to the size of the confocal microscopy optical head. Although the optical window enables a 400 x 400 μm² image, the optical head is too big to focus accurately on a precise area.
CONCLUSION
TG-CXL induces a greater flattening effect than C-CXL. It also produces a gradient in the biological response to treatment from the area of the cone to the surrounding area. Although longer follow-up in a larger cohort of patients is necessary, this technique seems to be a promising procedure that can result in greater flattening of the cone than C-CXL and consequently improved visual acuity.
Based on these results, TG-CXL seems to offer keratoconus patients a new option in personalized CXL with the potential for improvements in visual outcomes. It also holds the promise of further optimization in the future.
1. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620-627.
2. Sinha Roy A, Dupps WJ. Patient-specific computational modeling of keratoconus progression and differential responses to collagen cross-linking. Invest Ophthalmol Vis Sci. 2011;52:9174-9187.
3. Seiler TG, Fischinger I, Koller T, et al. Customized corneal cross-linking: one-year results. Am J Ophthalmol. 2016;166:14-21.
4. Cassagne M, Pierné K, Galiacy SD, et al. Customized topography guided corneal crosslinking for keratoconus: clinical results. J Refract Surg. [in press]



