In my opinion, the following is one of the most important questions in cataract surgery: Where should it be centered? But what does “centering cataract surgery” truly mean?
It is generally agreed that centering the IOL on or as close to the visual axis as possible is associated with the highest level of patient satisfaction postoperatively. I believe that there are two things to consider in answering the important question I have posed: the implant and the capsulorhexis.
The main reason surgeons should be just as purposeful in centering the capsulorhexis as the IOL itself is that contraction of the lens capsule can exert vector forces on the IOL that can move the optic out of position if proper surgical technique has not been used to prevent the occurrence. We surgeons need to understand that we are in control of long-term implant centration and that the manner in which we make the capsulorhexis has a tremendous impact on surgical outcomes.
Prevent Complications and Optimize Outcomes
The interaction of the IOL with the capsular opening can play a large role in minimizing irregular and aggressive capsular contraction vector forces.1 Multiple studies have shown the negative effects of allowing the apposition of the anterior capsular edge onto the posterior capsule near the posterior surface of the IOL optic. It can lead to fusion of the anterior capsule to the posterior capsule that can progress over time and create a gradual extrusion of the implant from its centered, physiologically tilted position into a decentered, abnormally tilted, and potentially visually disturbing position.2 I call this capsular fusion syndrome. Preventing this progressive, fibrotic anterior-to-posterior capsular adhesion is one of my main goals in cataract surgery.
If the opening is too small, capsular fibrosis and phimosis can become an issue. They can cause an anteroposterior IOL shift or IOL decentration over time because of asymmetric shrinkage of the capsular bag.3,4 If the capsular opening is too large or decentered, the anterior capsule that is now peripheral to the optic can fuse to the posterior capsule and create an aggressive capsular fibrosis that gradually displaces, decenters, and abnormally tilts the lens.5 Multiple studies have also demonstrated that an implant that is too large or small can have a negative impact on refractive outcomes and promote decentration.6,7
To minimize problems induced by the capsular opening, including fusion of the anterior and posterior capsules, I prioritize creating a well-centered, round, and precisely sized opening (ideally 5 mm) to overlap the anterior edge of the 6-mm optic for 360º so that capsular contraction keeps the implant’s optic where I placed it. There are basically two forms of posterior capsular opacification (PCO), a less aggressive pearl type and a more aggressive fibrotic type.5 A bonus of achieving 360º of capsular overlap is that a less aggressive form of PCO occurs that either doesn’t require an Nd:YAG laser capsulotomy or requires one using much less energy.
Preferred Technique for Centering the Capsulorhexis
My preferred technique for centering cataract surgery, both the implant and capsular opening, has been described.8 Beautiful, precise, and reproducible Purkinje light reflexes are often underutilized, despite how helpful and well worth learning they are. Of the four Purkinje images, the two that I find the most useful are the first Purkinje image (P1) and the fourth Purkinje image (P4; Figure 1).8 Others have nicely described centering cataract surgery on P1, also known as the subject-fixated coaxially sighted corneal light reflex.9 Centering the optic and capsulotomy on P1 involves the use of coaxial microscope optics, surgeon-instructed patient fixation on a coaxial light, and either a manual corneal optical zone marker (I use a 6.0 marker and go just inside of it to achieve a 5-mm opening) or the Zepto Capsulotomy System (Centricity Vision; Figure 2).8 If patient fixation on the coaxial light is precise, centering on P1 can be very accurate. Unfortunately, patient fixation is often negatively affected by sedation or patient inconsistency in looking at the bright light (Figure 3). Having a technique to serve as a surrogate for patient fixation is therefore helpful.8
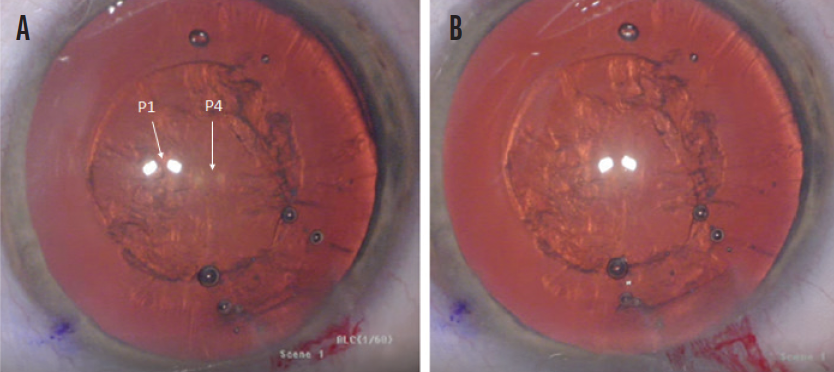
Figure 1. Misaligned P1 and P4 images where capsulotomy centration cannot be assessed (A) and a well-centered, round, and precisely sized capsulotomy with proper alignment of the P1 and P4 images (B).
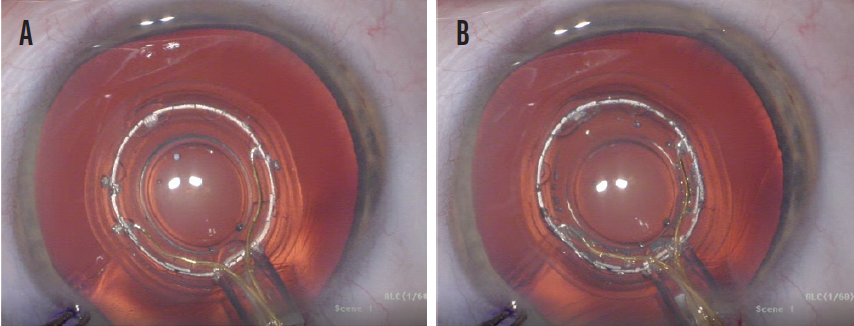
Figure 2. Misaligned P1 and P4 images and an improperly centered Zepto capsulotomy device (A). A properly centered Zepto capsulotomy device with properly aligned P1 and P4 images (B).
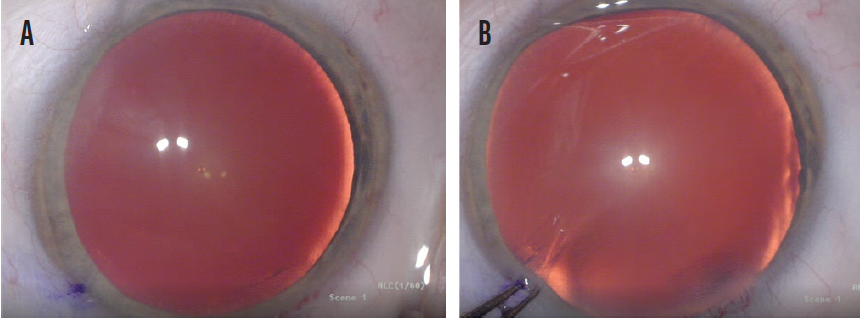
Figure 3. Misaligned P1 and P4 images due to imprecise patient fixation on the coaxial light (A) and proper alignment of the P1 and P4 images following manual adjustment of the eye’s position (B).
My preferred method of supplementing patient fixation is based on the fact that, when a patient fixates on a bright light, a unique, reproducible, and accurate relationship between their P1 and P4 images can be identified. If a patient is having difficulty looking at the light, I can manually adjust eye position with .12 forceps to align P1 and P4 and achieve a close approximation of the patient’s visual axis. I can then center the capsulotomy on these closely aligned images (Figure 4). Subtle variations in the degree of P1-P4 overlap at fixation are apparent among patients, reflecting their individual ocular anatomy.
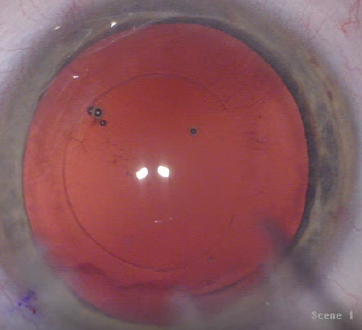
Figure 4. A well-centered, round, and precisely sized capsulotomy aligned to the P1 and P4 images.
The Purkinje method of centering the capsulotomy works well in conjunction with techniques that allow patient fixation or Purkinje image utilization (Figure 5). It is the best method I have found to achieve long-term centration of the implant and minimize the chance of fibrotic PCO by preventing capsular fusion syndrome.
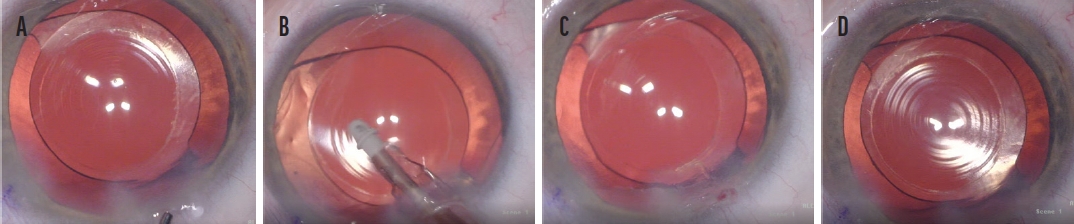
Figure 5. Misaligned P1 and P4 images in an eye in which the IOL is not properly centered on the visual axis (A). An I/A tip was used to rotate the IOL to a more centered position on the visual axis within the capsular bag (B). IOL centration cannot be assessed following the rotation maneuver until the P1 and P4 images are aligned (C). P1 and P4 are aligned and well centered on the optic, with 360º of anterior capsular overlap and IOL centration following the rotation maneuver can now be reassessed (D).
Conclusion
It is essential to center the capsulorhexis over a dilated pupil and achieve 360º overlap with the implant. Using the Purkinje images helps me to achieve this complete overlap with greater success than I would achieve by centering the capsulorhexis on the pupil. One reason is that the pupil typically is not centered over the crystalline or artificial lens. Furthermore, the crystalline lens is not perfectly round, and there are unequal lens zonular vector forces. Using the Purkinje image with the patient fixating on the light (or using the P1-P4 aligned image) helps me to rotate the lens to that optimal position centered as close to the visual axis as possible. These principles are important with any lens implant but particularly for aspheric monofocal, extended depth of focus, and multifocal IOLs.
1. Andley UP. The lens epithelium: focus on the expression and function of the alpha-crystallin chaperones. Int J Biochem Cell Biol. 2008;40(3):317-323.
2. Carlson AN, Stewart WC, Tso PC. Intraocular lens complications requiring removal or exchange. Surv Ophthalmol. 1998;42:417-440.
3. Qatarneh D, Hau S, Tuft S. Hyperopic shift from posterior migration of hydrophilic acrylic intraocular lens optic. J Cataract Refract Surg. 2010;36(1):161-163.
4. Zaugg B, Werner L, Neuhann T, et al. Clinicopathologic correlation of capsulorhexis phimosis with anterior flexing of single-piece hydrophilic acrylic intraocular lens haptics. J Cataract Refract Surg. 2010;36(9):1605-1609.
5. Apple DJ, Solomon KD, Tetz MR. Posterior capsule opacification. Surv Ophthalmol. 1992;37(2):73-116.
6. Li S, Hu Y, Guo R, et al. The effects of different shapes of capsulorrhexis on postoperative refractive outcomes and the effective position of the intraocular lens in cataract surgery. BMC Ophthalmol. 2019;21;19(1):59.
7. Okada M, Hersh D, Paul E, van der Straaten D. Effect of centration and circularity of manual capsulorrhexis on cataract surgery refractive outcomes. Ophthalmology. 2014;121(3):763-770.
8. Thompson V. Streamlined method for anchoring cataract surgery and intraocular lens centration on the patient’s visual axis. J Cataract Refract Surg. 2018;44:528-533.
9. Chang DH, Waring GO. The subject-fixated coaxially sighted corneal light reflex: a clinical marker for centration of refractive treatments and devices. Am J Ophthalmol. 2014;158:863-874.